Can the Treatment of Normal-Pressure Hydrocephalus Induce Normal-Tension Glaucoma? A Narrative Review of a Current Knowledge
Abstract
:1. Introduction
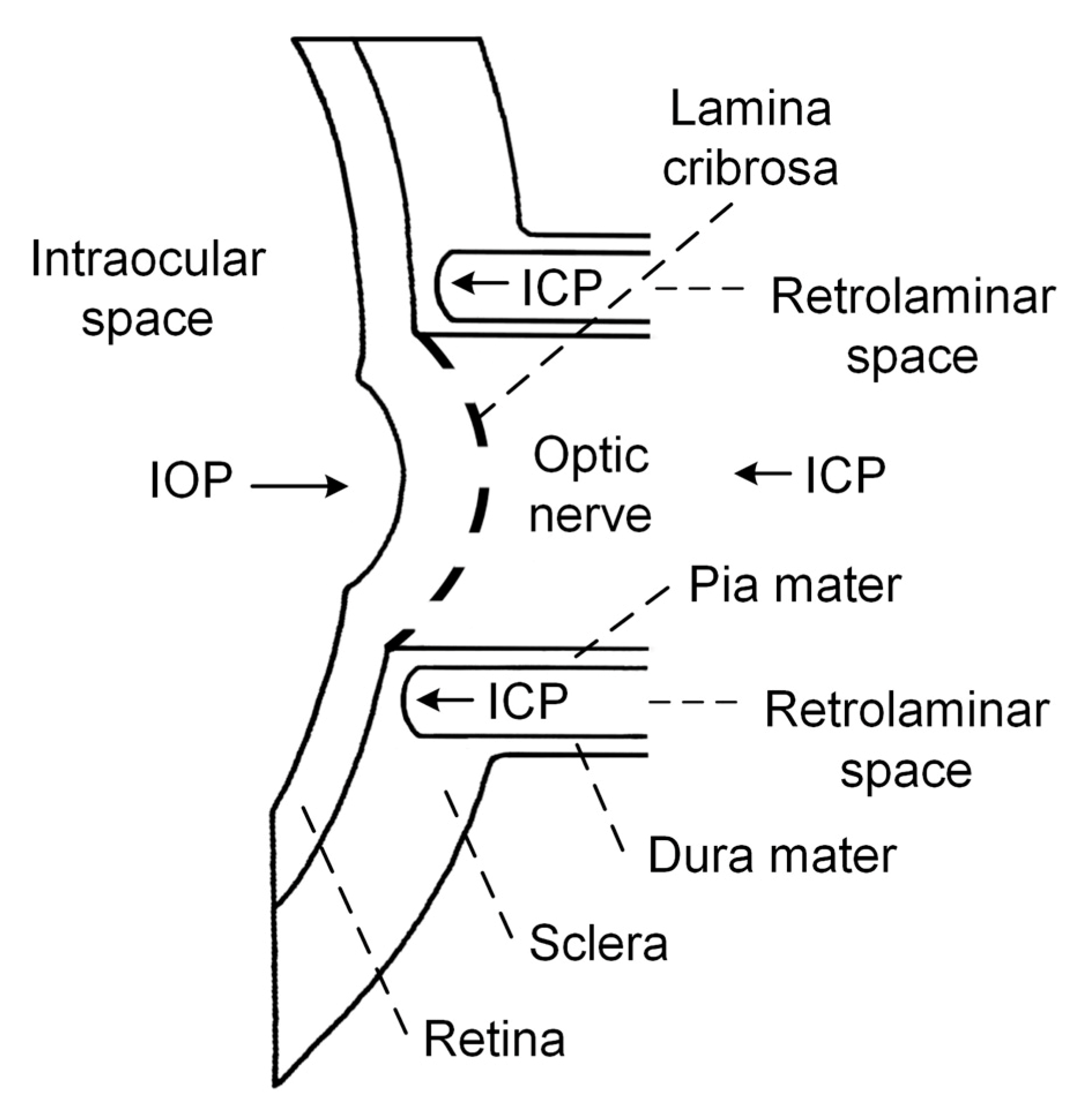
1.1. Pathophysiologic Mechanism of ON Damage in NTG
1.2. Association between NPH and NTG
1.3. Prospective Studies
1.4. Retrospective Studies
1.5. Case Reports
1.6. Review Papers Including Medical Hypotheses
2. Results
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kotagal, V.; Walkowiak, E.; Heth, J.A. Serious adverse events following Normal Pressure Hydrocephalus surgery. Clin. Neurol. Neurosurg. 2018, 170, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Lalou, A.D.; Czosnyka, M.; Donnelly, J.; Pickard, J.D.; Nabbanja, E.; Keong, N.C.; Garnett, M.; Czosnyka, Z.H. Cerebral autoregulation, cerebrospinal fluid outflow resistance, and outcome following cerebrospinal fluid diversion in normal pressure hydrocephalus. J. Neurosurg. JNS 2018, 130, 154–162. [Google Scholar] [CrossRef]
- Shprecher, D.; Schwalb, J.; Kurlan, R. Normal pressure hydrocephalus: Diagnosis and treatment. Curr. Neurol. Neurosci. Rep. 2008, 8, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, S. FSI simulation of CSF hydrodynamic changes in a large population of non-communicating hydrocephalus patients during treatment process with regard to their clinical symptoms. PLoS ONE 2018, 13, e0196216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czosnyka, Z.; Czosnyka, M. Long-term monitoring of intracranial pressure in normal pressure hydrocephalus and other CSF disorders. Acta Neurochir. (Wien.) 2017, 159, 1979–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wostyn, P.; Audenaert, K.; De Deyn, P.P. Alzheimer’s disease-related changes in diseases characterized by elevation of intracranial or intraocular pressure. Clin. Neurol. Neurosurg. 2008, 110, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Kashkoush, A.; McDowell, M.M.; Lariviere, W.R.; Ismail, N.; Friedlander, R.M. Comparative durability and costs analysis of ventricular shunts. J. Neurosurg. JNS 2018, 130, 1252–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, A.H.; Due-Tønnessen, B.J.; Lundar, T.; Lindegaard, K.-F. Cerebrospinal fluid (CSF) shunting and ventriculocisternostomy (ETV) in 400 pediatric patients. Shifts in understanding, diagnostics, case-mix, and surgical management during half a century. Child’s Nerv. Syst. 2017, 33, 259–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokhari, R.F.; Baeesa, S.S. Does the treatment of normal pressure hydrocephalus put the retinal ganglion cells at risk? A brief literature review and novel hypothesis. Med. Hypotheses 2013, 81, 686–689. [Google Scholar] [CrossRef]
- Wostyn, P.; Audenaert, K.; De Deyn, P.P. High Occurrence Rate of Glaucoma Among Patients with Normal Pressure Hydrocephalus. J. Glaucoma 2010, 19, 225–226. [Google Scholar] [CrossRef] [PubMed]
- Pircher, A.; Montali, M.; Wostyn, P.; Pircher, J.; Berberat, J.; Remonda, L.; Killer, H.E. Impaired cerebrospinal fluid dynamics along the entire optic nerve in normal-tension glaucoma. Acta Ophthalmol. 2018, 96, e562–e569. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhai, R.; Zong, Y.; Kong, X.; Jiang, C.; Sun, X.; He, Y.; Li, X. Comparison of retinal microvascular changes in eyes with high-tension glaucoma or normal-tension glaucoma: A quantitative optic coherence tomography angiographic study. Graefe’s Arch. Clin. Exp. Ophthalmol. = Albr. von Graefes Arch. fur Klin. Exp. Ophthalmol. 2018, 256, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Plange, N.; Remky, A.; Arend, O. Colour Doppler imaging and fluorescein filling defects of the optic disc in normal tension glaucoma. Br. J. Ophthalmol. 2003, 87, 731–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, T.; Utsunomiya, K.; Ota, H.; Ogura, Y.; Narabayashi, I.; Ikeda, T. Comparative study of cerebral blood flow in patients with normal-tension glaucoma and control subjects. Am. J. Ophthalmol. 2006, 141, 394–396. [Google Scholar] [CrossRef]
- Fan, N.; Wang, P.; Tang, L.; Liu, X. Ocular Blood Flow and Normal Tension Glaucoma. Biomed Res. Int. 2015, 12, 308505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, R.M.; Yang, A.; Brahma, V.; Martone, J.F. Management of Blood Pressure in Patients with Glaucoma. Curr. Cardiol. Rep. 2017, 19, 109. [Google Scholar] [CrossRef]
- Hayreh, S.S.; Zimmerman, M.B.; Podhajsky, P.; Alward, W.L. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am. J. Ophthalmol. 1994, 117, 603–624. [Google Scholar] [CrossRef]
- Grus, F.H.; Joachim, S.C.; Wuenschig, D.; Rieck, J.; Pfeiffer, N. Autoimmunity and glaucoma. J. Glaucoma 2008, 17, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Wax, M.B. The case for autoimmunity in glaucoma. Exp. Eye Res. 2011, 93, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Tsai, J.C.; Kolko, M. The role of inflammation in the pathogenesis of glaucoma. Surv. Ophthalmol. 2013, 58, 311–320. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, E.M.; Kim, G.A.; Kwak, S.W.; Kim, J.M.; Bae, H.W.; Seong, G.J.; Kim, C.Y. Three Toxic Heavy Metals in Open-Angle Glaucoma with Low-Teen and High-Teen Intraocular Pressure: A Cross-Sectional Study from South Korea. PLoS ONE 2016, 11, e0164983. [Google Scholar] [CrossRef]
- Volkov, V. V Essential element of the glaucomatous process neglected in clinical practice. Oftalmol. Zh. 1976, 31, 500–504. [Google Scholar]
- Morgan-Davies, J.; Taylor, N.; Hill, A.R.; Aspinall, P.; O’Brien, C.J.; Azuara-Blanco, A. Three dimensional analysis of the lamina cribrosa in glaucoma. Br. J. Ophthalmol. 2004, 88, 1299–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCulley, T.J.; Chang, J.R.; Piluek, W.J. Intracranial pressure and glaucoma. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 2015, 35 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Wostyn, P.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P.; De Groot, V. A new glaucoma hypothesis: A role of glymphatic system dysfunction. Fluids Barriers CNS 2015, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallina, P.; Savastano, A.; Becattini, E.; Orlandini, S.; Scollato, A.; Rizzo, S.; Carreras, G.; Di Lorenzo, N.; Porfirio, B. Glaucoma in patients with shunt-treated normal pressure hydrocephalus. J. Neurosurg. JNS 2017, 129. [Google Scholar] [CrossRef] [Green Version]
- Yablonski, M.E.; Ritch, R.; Pokorny, K.S. Effect of decreased intracranial-pressure on optic disk. In Investigative Ophthalmology & Visual Science; Lippincott-Raven Publ 227 East Washington Sq: Philadelphia, PA, USA, 1979; p. 165. [Google Scholar]
- Berdahl, J.P.; Fautsch, M.P.; Stinnett, S.S.; Allingham, R.R. Intracranial pressure in primary open angle glaucoma, normal tension glaucoma, and ocular hypertension: A case-control study. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5412–5418. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Jonas, J.B.; Tian, G.; Zhen, Y.; Ma, K.; Li, S.; Wang, H.; Li, B.; Zhang, X.; Wang, N. Cerebrospinal Fluid Pressure in Glaucoma. A Prospective Study. Ophthalmology 2010, 117, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Siaudvytyte, L.; Januleviciene, I.; Ragauskas, A.; Bartusis, L.; Meiliuniene, I.; Siesky, B.; Harris, A. The difference in translaminar pressure gradient and neuroretinal rim area in glaucoma and healthy subjects. J. Ophthalmol. 2014, 2014, 937360. [Google Scholar] [CrossRef] [PubMed]
- Lindén, C.; Qvarlander, S.; Jóhannesson, G.; Johansson, E.; Östlund, F.; Malm, J.; Eklund, A. Normal-Tension Glaucoma Has Normal Intracranial Pressure: A Prospective Study of Intracranial Pressure and Intraocular Pressure in Different Body Positions. Ophthalmology 2018, 125, 361–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jóhannesson, G.; Eklund, A.; Lindén, C. Intracranial and Intraocular Pressure at the Lamina Cribrosa: Gradient Effects. Curr. Neurol. Neurosci. Rep. 2018, 18, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wostyn, P.; De Groot, V.; Van Dam, D.; Audenaert, K.; Killer, H.E.; De Deyn, P.P. The Glymphatic Hypothesis of Glaucoma: A Unifying Concept Incorporating Vascular, Biomechanical, and Biochemical Aspects of the Disease. Biomed. Res. Int. 2017, 2017, 5123148. [Google Scholar] [CrossRef] [PubMed]
- Mori, E.; Ishikawa, M.; Kato, T.; Kazui, H.; Miyake, H.; Miyajima, M.; Nakajima, M.; Hashimoto, M.; Kuriyama, N.; Tokuda, T.; et al. Guidelines for management of idiopathic normal pressure hydrocephalus: Second edition. Neurol. Med. Chir. 2012, 52, 775–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, H.; Kajimoto, Y.; Tsuji, M.; Ukita, T.; Tucker, A.; Ohmura, T. Development of a Quick Reference Table for Setting Programmable Pressure Valves in Patients With Idiopathic Normal Pressure Hydrocephalus. Neurol. Med. Chir. 2008, 48, 427–432; discussion 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Fu, J.; Hou, R.; Liu, K.; Jonas, J.B.; Wang, H.; Chen, W.; Li, Z.; Sang, J.; Zhang, Z.; et al. Optic neuropathy induced by experimentally reduced cerebrospinal fluid pressure in monkeys. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3067–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudolph, D.; Sterker, I.; Graefe, G.; Till, H.; Ulrich, A.; Geyer, C. Visual field constriction in children with shunt-treated hydrocephalus. J. Neurosurg. Pediatr. 2010, 6, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Singh, K. Glaucomatous disease in patients with normal pressure hydrocephalus. J. Glaucoma 2009, 18, 243–246. [Google Scholar] [CrossRef]
- Heinsbergen, I.; Rotteveel, J.; Roeleveld, N.; Grotenhuis, A. Outcome in shunted hydrocephalic children. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2002, 6, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Drucker, M.D.; Louis, K.M.; Richards, D.W. Progression of Normal-Tension Glaucoma After Ventriculoperitoneal Shunt to Decrease Cerebrospinal Fluid Pressure. J. Glaucoma 2016, 25, e50–e52. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Ratnarajan, G.; Kerr, R.S.; Salmon, J.F. Juvenile-onset Normal Tension Glaucoma From Chronic, Recurrent Low Cerebrospinal Fluid Pressure. J. Glaucoma 2016, 25, e738–e740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wostyn, P.; Van Dam, D.; De Deyn, P.P. Intracranial pressure and glaucoma: Is there a new therapeutic perspective on the horizon? Med. Hypotheses 2018, 118, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Ichibayashi, R.; Suzuki, G.; Yokomuro, H.; Seiki, Y.; Sase, S.; Kishi, T. Consideration of the Intracranial Pressure Threshold Value for the Initiation of Traumatic Brain Injury Treatment: A Xenon CT and Perfusion CT Study. Neurocrit. Care 2017, 27, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Stocchetti, N.; Poole, D.; Okonkwo, D.O. Intracranial pressure thresholds in severe traumatic brain injury: We are not sure: Prudent clinical practice despite dogma or nihilism. Intensive Care Med. 2018, 44, 1321–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.J.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Adams and Victor’s Principles of Neurology; Ropper, A.H.; Brown, R.H. (Eds.) Adams and Victors Principles of Neurology; McGraw-Hill Medical Pub. Division: New York, NY, USA, 2005; ISBN 9780071416207. [Google Scholar]
- Czosnyka, M.; Pickard, J.D. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Antes, S.; Stadie, A.; Müller, S.; Linsler, S.; Breuskin, D.; Oertel, J. Intracranial Pressure-Guided Shunt Valve Adjustments with the Miethke Sensor Reservoir. World Neurosurg. 2018, 109, e642–e650. [Google Scholar] [CrossRef] [PubMed]
- Raboel, P.H.; Bartek, J.J.; Andresen, M.; Bellander, B.M.; Romner, B. Intracranial Pressure Monitoring: Invasive versus Non-Invasive Methods-A Review. Crit. Care Res. Pract. 2012, 2012, 950393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siaudvytyte, L.; Januleviciene, I.; Ragauskas, A.; Bartusis, L.; Siesky, B.; Harris, A. Update in intracranial pressure evaluation methods and translaminar pressure gradient role in glaucoma. Acta Ophthalmol. 2015, 93, 9–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragauskas, A.; Matijosaitis, V.; Zakelis, R.; Petrikonis, K.; Rastenyte, D.; Piper, I.; Daubaris, G. Clinical assessment of noninvasive intracranial pressure absolute value measurement method. Neurology 2012, 78, 1684–1691. [Google Scholar] [CrossRef]
- Ragauskas, A.; Bartusis, L.; Piper, I.; Zakelis, R.; Matijosaitis, V.; Petrikonis, K.; Rastenyte, D. Improved diagnostic value of a TCD-based non-invasive ICP measurement method compared with the sonographic ONSD method for detecting elevated intracranial pressure. Neurol. Res. 2014, 36, 607–614. [Google Scholar] [CrossRef]
- Bershad, E.M.; Anand, A.; DeSantis, S.M.; Yang, M.; Tang, R.A.; Calvillo, E.; Malkin-Gosdin, L.; Foroozan, R.; Damani, R.; Maldonado, N.; et al. Clinical Validation of a Transcranial Doppler-Based Noninvasive Intracranial Pressure Meter: A Prospective Cross-Sectional Study. World Neurosurg. 2016, 89, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.; Siesky, B.; Wirostko, B. Cerebral blood flow in glaucoma patients. J. Glaucoma 2013, 22 (Suppl. S5), S46–S48. [Google Scholar] [CrossRef] [PubMed]
- Kumpaitiene, B.; Svagzdiene, M.; Sirvinskas, E.; Adomaitiene, V.; Petkus, V.; Zakelis, R.; Krakauskaite, S.; Chomskis, R.; Ragauskas, A.; Benetis, R. Cerebrovascular autoregulation impairments during cardiac surgery with cardiopulmonary bypass are related to postoperative cognitive deterioration: Prospective observational study. Minerva Anestesiol. 2019, 85, 594–603. [Google Scholar] [CrossRef] [PubMed]
| Study | Method | Sample Size | Age, Years | Study Group | Control Group | Time Frame | Glaucomatous Damage |
|---|---|---|---|---|---|---|---|
| Gallina P, et al. [26] | Prospective study | 12 males, 10 females | Range of 68–87 years | 22 adult | - | 6 months | 9 patients |
| Yang D, et al. [36] | Prospective study | 9 male Rhesus monkeys | An average age of 6 years | 4 Rhesus monkeys | 5 Rhesus monkeys | 12 months | 3 Rhesus monkeys |
| Rudolph D, et al. [37] | Prospective study | 32 boys, 24 girls | An average age of 15 years | 56 children | - | 12 months | 13 patients |
| Chang TC and Singh K. [38]. | Retrospective study | 67 males, 77 females | An average age of 75 years | 72 adult | 72 adult | 132 months | 13 patients |
| Heinsbergen I, et al. [39] | Retrospective study | 67 males, 52 females | Range of 1–5 years | 119 children | - | 96 months | N/S |
| Chen BH, et al. [40] | Case report | 1 female | 93 | 1 adult | - | 162 months | 1 patient |
| Yusuf IH, et al. [41] | Case report | 1 male | 27 | 1 adult | - | 300 months | 1 patient |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamarat, Y.; Bartusis, L.; Deimantavicius, M.; Lucinskas, P.; Siaudvytyte, L.; Zakelis, R.; Harris, A.; Mathew, S.; Siesky, B.; Janulevicienė, I.; et al. Can the Treatment of Normal-Pressure Hydrocephalus Induce Normal-Tension Glaucoma? A Narrative Review of a Current Knowledge. Medicina 2021, 57, 234. https://doi.org/10.3390/medicina57030234
Hamarat Y, Bartusis L, Deimantavicius M, Lucinskas P, Siaudvytyte L, Zakelis R, Harris A, Mathew S, Siesky B, Janulevicienė I, et al. Can the Treatment of Normal-Pressure Hydrocephalus Induce Normal-Tension Glaucoma? A Narrative Review of a Current Knowledge. Medicina. 2021; 57(3):234. https://doi.org/10.3390/medicina57030234
Chicago/Turabian StyleHamarat, Yasin, Laimonas Bartusis, Mantas Deimantavicius, Paulius Lucinskas, Lina Siaudvytyte, Rolandas Zakelis, Alon Harris, Sunu Mathew, Brent Siesky, Ingrida Janulevicienė, and et al. 2021. "Can the Treatment of Normal-Pressure Hydrocephalus Induce Normal-Tension Glaucoma? A Narrative Review of a Current Knowledge" Medicina 57, no. 3: 234. https://doi.org/10.3390/medicina57030234







