What We Should Consider in Point of Care Blood Glucose Test; Current Quality Management Status of a Single Institution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Monitoring
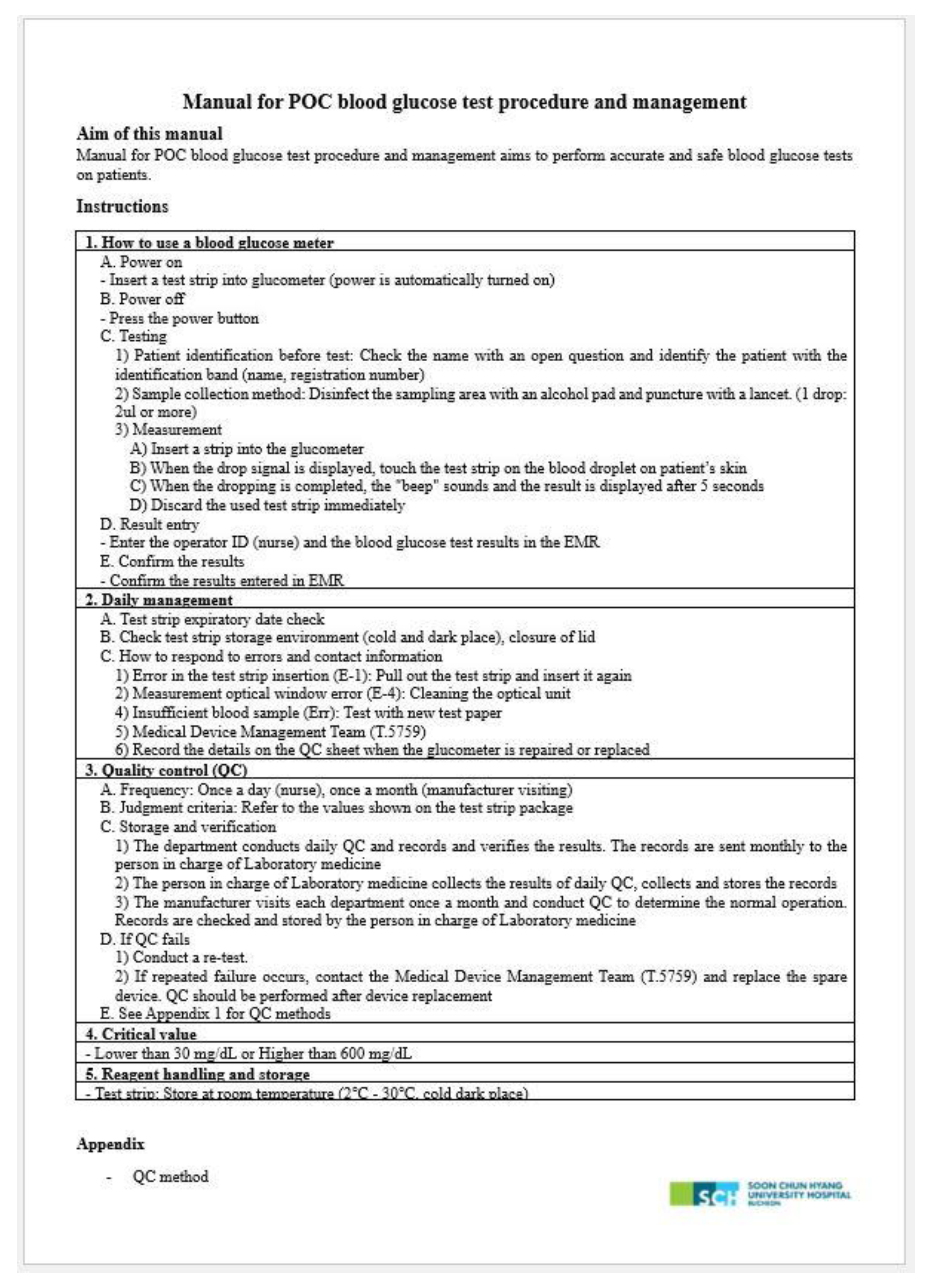
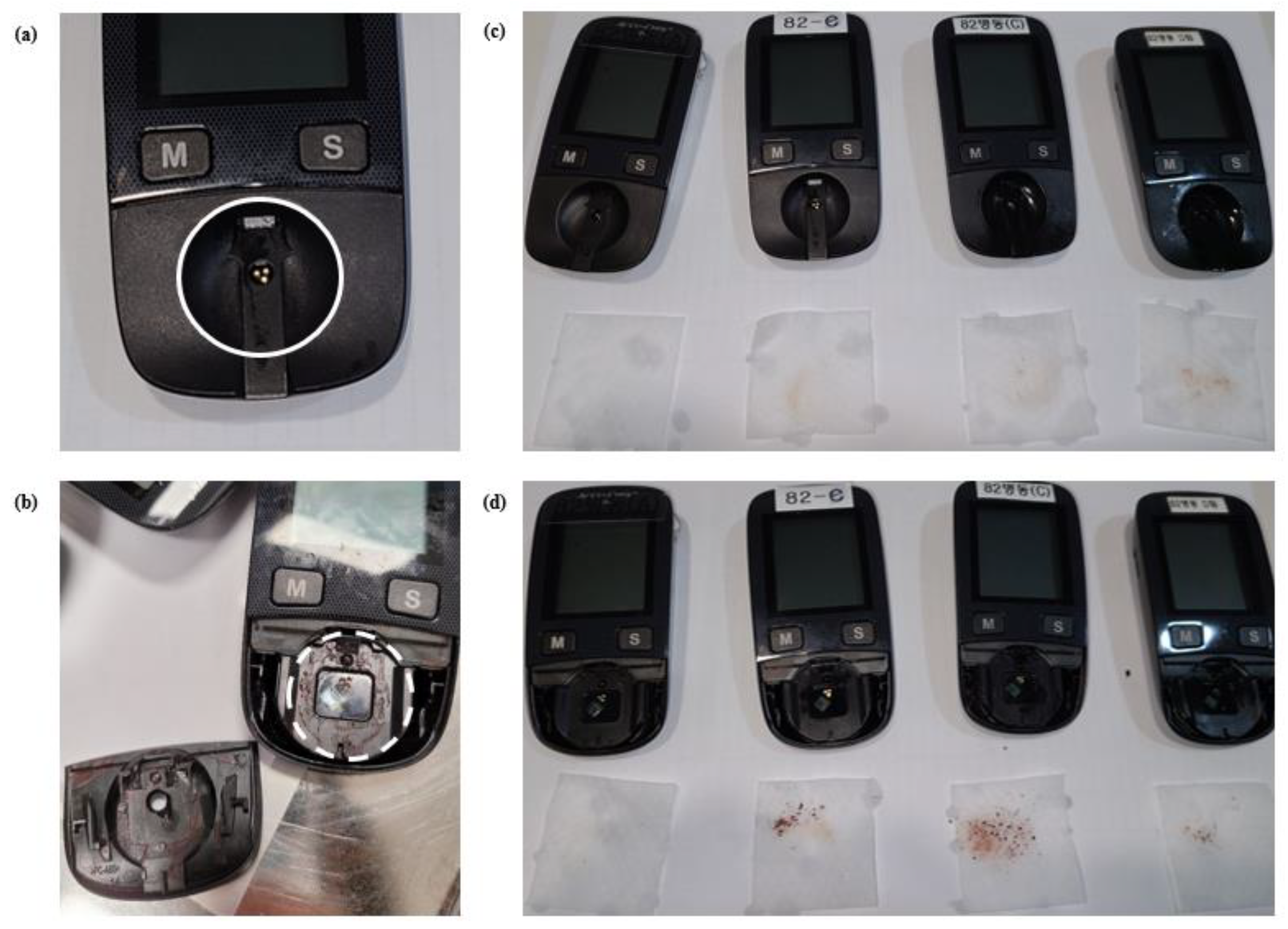
- Glucometer: Test Strip Inlet Cleaning, Measurement Optical Window Cleaning, and Storage.
- 2.
- Test strip kit: serial and lot number of kits, opening date, expiration date, and sealing status were checked. Only the test strip kit, which did not move at all when the lid was pressed down, was determined as “sealed”.
- 3.
- QC material: Opening date and expiration date.
- 4.
- Quality assurance program: recording of information about strip and QC material including recording format, review QC and troubleshooting results.
- 5.
- Others: type of samples, sampling method, blood applying method and result recording.
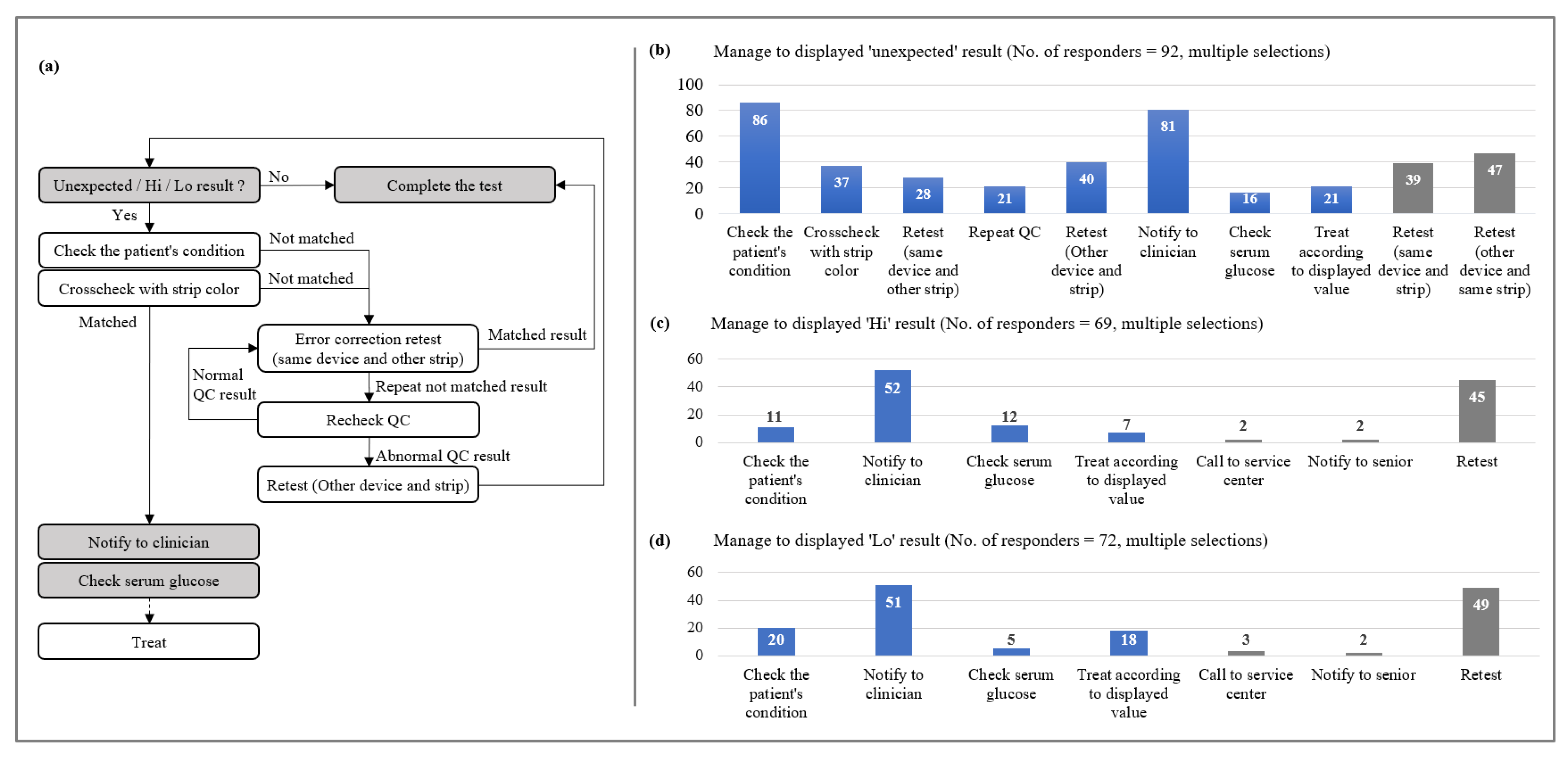
2.2. Manage Sequence to Specific Results
2.3. Glucose Level Data Analysis
- When the results had decimal points, symbol and letter, we classified as “atypical BST results”. The percentage of these results for the entire BST menu data was calculated.
- The retrospective data of blood glucose test of atypical BST results was compared with the results of the central laboratory or POC glucometer received approximately three minutes before or after the time the atypical BST result was recorded.
- Each department team showed examples of atypical BST results and asked if they had ever recorded the results and what they meant.
2.4. Statistical Methods
3. Results
3.1. Monitoring
3.1.1. Glucometer
3.1.2. Test Strip Kit
3.1.3. QC Material
3.1.4. Quality Assurance Program
3.1.5. Others
3.2. Manage Sequence to Specific Results
- Unexpected values response: More than 80% of respondents said they would check the patient’s condition (93.5%, 86/92) and report it to the clinician (88.0%, 81/92) if unexpected results were displayed.
- Measurable range values response: The most common response was to notify the clinician (75.4%, 52/69) followed by retesting (65.2%, 45/69).
- Measurable range values response: The most common response was to notify the clinician (70.8%, 51/72) followed retest (68.1%, 49/72).
3.3. Glucose Level Data Analysis
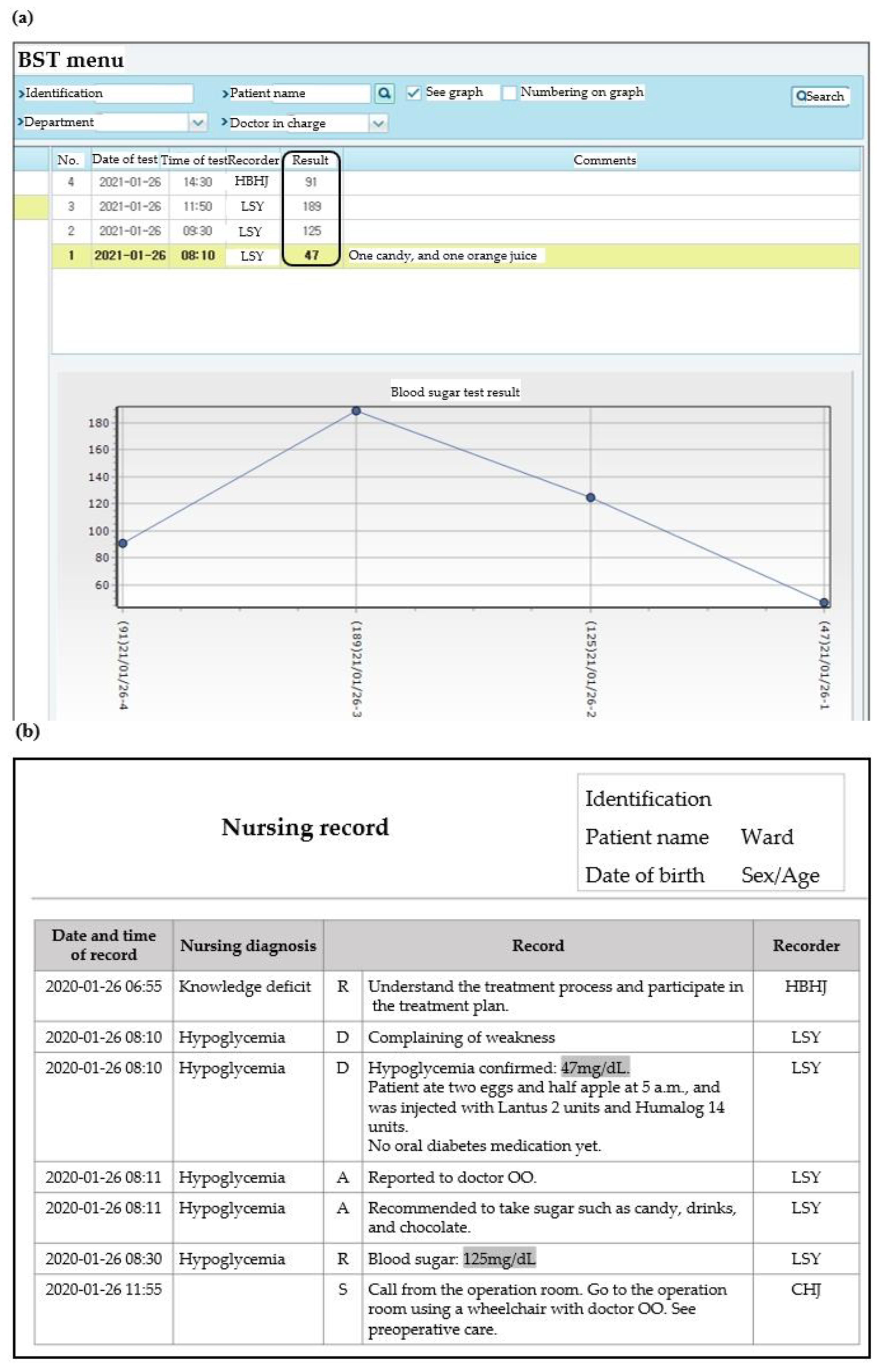
- The total number of test strip ordered during the analysis period was 768,450 (Table 3). The BST menu dataset contained 572,027 results for 13,786 patients from 23 departments. A total of 4568 atypical BST results were identified. Twenty-two of them were out of measurable range (20 results above 600 mg/dL and 2 results below 10 mg/dL), and the remaining 4546 results were recorded by symbol that could not be discussed alone, or by combination of numbers and symbols. The main types of symbols used were “.”, “,” “-”, “--,” and “---”.
- Twenty-two atypical BST results out of measurable range were compared with the results of the central laboratory or POC glucometer. For those below the measurable range, no other tests were performed simultaneously. A total of 10 results were above the measurable range results, two were identical to serum glucose results and seven were identical to arterial blood gas analysis (aBGA) results. One of the serum glucose tests was performed simultaneously, but the results were different with atypical BST results recorded on BST menu.
- Although atypical BST results were observed in 23 departments, only 10 teams among 64 teams belongs to previous 23 departments were aware of the meaning of the results recorded as symbols. In all the departments that did not report atypical results no one knew the meaning of the symbols. As for the meaning of the symbol, the most frequent answer was “not tested”.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, H.; Ko, D.H.; Kim, J.Q.; Song, S.H. Performance evaluation of the Piccolo xpress Point-of-care Chemistry Analyzer. Korean J. Lab. Med. 2009, 29, 430–438. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, G.; Dou, W. Portable and quantitative point-of-care monitoring of Escherichia coli O157: H7 using a personal glucose meter based on immunochromatographic assay. Biosens. Bioelectron. 2018, 107, 266–271. [Google Scholar] [CrossRef]
- St-Louis, P. Status of point-of-care testing: Promise, realities, and possibilities. Clin. Biochem. 2000, 33, 427–440. [Google Scholar] [CrossRef]
- Huang, Y.; Campbell, E.; Colbourne, B.; Power, J.; Randell, E. User competency is still a major factor affecting analytical performance of glucose meters in patient service. Clin. Biochem. 2019, 63, 66–71. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Notes from the field: Deaths from acute hepatitis B virus infection associated with assisted blood glucose monitoring in an assisted-living facility—North Carolina, August–October 2010. MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 182. [Google Scholar]
- Thompson, N.D.; Schaefer, M.K. “Never events”: Hepatitis B outbreaks and patient notifications resulting from unsafe practices during assisted monitoring of blood glucose, 2009–2010. J. Diabetes Sci. Technol. 2011, 5, 1396–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnell, O.; Kulzer, B.; Erbach, M. Hygiene and Disinfection of Blood Glucose Meters in Multi-patient Setting. Il G. AMD 2014, 17, 139–142. [Google Scholar]
- Ng, V. Point-of-care glucose meter testing in 2014: Infection prevention and regulatory challenges. Point Care 2014, 13, 84–87. [Google Scholar] [CrossRef]
- Tonyushkina, K.; Nichols, J.H. Glucose meters: A review of technical challenges to obtaining accurate results. J. Diabetes Sci. Technol. 2009, 3, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, M.J.; Shuster, J.J.; Schatz, D.; Melker, R.J. Adverse impact of temperature and humidity on blood glucose monitoring reliability: A pilot study. Diabetes Technol. Ther. 2007, 9, 1–9. [Google Scholar] [CrossRef]
- Baumstark, A.; Pleus, S.; Schmid, C.; Link, M.; Haug, C.; Freckmann, G. Lot-to-lot variability of test strips and accuracy assessment of systems for self-monitoring of blood glucose according to ISO 15197. J. Diabetes Sci. Technol. 2012, 6, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, G.B.B.; Christensen, N.G.; Thue, G.; Sandberg, S. Between-lot variation in external quality assessment of glucose: Clinical importance and effect on participant performance evaluation. Clin. Chem. 2005, 51, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Glucose Monitoring in Settings without Laboratory Support, 3rd ed.; CLSI Document POCT 13–ED3CE; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Clinical and Laboratory Standards Institute. Point-of-Care Blood Glucose Testing in Acute and Chronic Care Facilities; Approved Guideline; CLSI Document POCT 12–A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Kitchen, D.P.; Jennings, I.; Kitchen, S.; Woods, T.A.L.; Walker, I.D. Bridging the Gap between Point-of-Care Testing and Laboratory Testing in Hemostasis. Semin. Thromb. Hemost. 2015, 41, 272–278. [Google Scholar] [PubMed]
- Cantero, M.; Redondo, M.; Martín, E.; Callejón, G.; Hortas, M.L. Use of quality indicators to compare point-of-care testing errors in a neonatal unit and errors in a STAT central laboratory. Clin. Chem. Lab. Med. CCLM 2015, 53, 239–247. [Google Scholar] [CrossRef]
- O’Kane, M.J.; McManus, P.; McGowan, N.; Lynch, P.L.M. Quality error rates in point-of-care testing. Clin. Chem. 2011, 57, 1267–1271. [Google Scholar] [CrossRef] [Green Version]
- Plebani, M. The detection and prevention of errors in laboratory medicine. Ann. Clin. Biochem. 2010, 47, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Crook, M.A. Near patient testing and pathology in the new millennium. J. Clin. Pathol. 2000, 53, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Bock, J.L.; Vasek, J. The flip side of point-of-care testing: Opportunities for postanalytical error. Point Care 2011, 10, 174–175. [Google Scholar] [CrossRef]
- Carraro, P.; Plebani, M. Post-analytical errors with portable glucose meters in the hospital setting. Clin. Chim. Acta 2009, 404, 65–67. [Google Scholar] [CrossRef]
- Mays, J.A.; Mathias, P.C. Measuring the rate of manual transcription error in outpatient point-of-care testing. J. Am. Med. Inform. Assoc. 2019, 26, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Meier, F.A.; Jones, B.A. Point-of-care testing error: Sources and amplifiers, taxonomy, prevention strategies, and detection monitors. Arch. Pathol. Lab. Med. 2005, 129, 1262–1267. [Google Scholar] [CrossRef]
- Lee-Lewandrowski, E.; Corboy, D.; Lewandrowski, K.; Sinclair, J.; McDermot, S.; Benzer, T.I. Implementation of a point-of-care satellite laboratory in the emergency department of an academic medical center: Impact on test turnaround time and patient emergency department length of stay. Arch. Pathol. Lab. Med. 2003, 127, 456–460. [Google Scholar] [CrossRef]
- Shaw, J.L.V. Practical challenges related to point of care testing. Pract. Lab. Med. 2016, 4, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Larsson, A.; Greig-Pylypczuk, R.; Huisman, A. The state of point-of-care testing: A European perspective. Upsala J. Med. Sci. 2015, 120, 1–10. [Google Scholar] [CrossRef]
- Liikanen, E.; Lehto, L. Training of nurses in point-of-care testing: A systematic review of the literature. J. Clin. Nurs. 2013, 22, 2244–2252. [Google Scholar] [CrossRef]
- Lehto, L.; Liikanen, E.; Melkko, T.; Ebeling, T.; Kouri, T. An interactive two-step training and management model of point-of-care glucose testing in northern Finland. Int. J. Circumpolar Health 2011, 70, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L. Quality of Glucose Measurement with Blood Glucose Meters at the Point-of-Care: Relevance of Interfering Factors. Diabetes Technol. Ther. 2010, 12, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Tang Friesner, C.; Meyer, J.; Nippak, P. Glucose point-of-care meter operators competency: An assessment checklist. Pract. Lab. Med. 2020, 20, e00157. [Google Scholar] [CrossRef]
- Lee, J.H.; Cha, Y.J. Performance Evaluation of BAROZEN H, a Networking Blood Glucose Monitoring System for Medical Institutions. Lab. Med. Online 2015, 5, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Ahn, S.; Yim, J.; Cheon, Y.; Jeong, S.H.; Lee, S.; Kim, J. Influence of Vitamin C and Maltose on the Accuracy of Three Models of Glucose Meters. Ann. Lab. Med. 2016, 36, 271–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| General Characteristics of the Institution | ||
| Number of beds | 928 | |
| Average daily outpatients | 3093 | |
| Average daily inpatients | 801 | |
| Total number of glucometers | 127 | |
| Number of glucometers monitored in this study | 124 | |
| Average daily test strip usage in 2019 | 1403 | |
| Total number of departments performing blood glucose tests | 50 | |
| Number of departments monitored in this study | 47 | |
| Distribution of Departments Involved in This Study | ||
| Ward (N = 26) | Outpatient clinic (N = 11) | Special department (N = 10) |
| Delivery room Twenty general wards Four intensive care units Neonates’ room | Allergy and respiratory medicine Cardiology Comprehensive check-up center Endocrinology Family medicine Gastroenterology General check-up center Nephrology Oncology and hematology Pain medicine Pediatrics | Bronchoscope Cardiovascular center Central injection CT preparation Day operation center Dialysis Emergency room Nuclear medicine injection Nursing department Operating room |
| Glucometer | |||||||
| Total no. of device | 124 | ||||||
| Cleaning | Test strip inlet | Clean | Not clean | ||||
| 67 | 54.0% | 57 | 46.0% | ||||
| Measurement optical window | Clean | Not clean | |||||
| 55 | 44.4% | 69 | 55.6% | ||||
| Test Strip Kit | |||||||
| Opening date notation | Yes | No | |||||
| 3 | 2.8% | 104 | 97.2% | ||||
| Sealing | Sealed | Not sealed | |||||
| 48 | 44.9% | 59 | 55.1% | ||||
| QC Material | |||||||
| Opening date notation | Yes | No | |||||
| 26 | 54.2% | 22 | 45.8% | ||||
| Other | |||||||
| Types of samples used (duplicated check) | Capillary blood * | Venous blood | Arterial blood | ||||
| 46 | 97.9% | 31 | 66.0% | 8 | 17.0% | ||
| CSF | Urine | ||||||
| 5 | 10.6% | 1 | 2.1% | ||||
| Capillary blood sampling method | No squeezing | Squeezing | |||||
| 3 | 6.4% | 43 | 91.5% | ||||
| Test strip and lancing device deposition | Bring disposal container | Using tray | |||||
| 44 | 93.6% | 3 | 6.4% | ||||
| Department | Approximate Test Strip Usage | No. of Results | No. of Respondents | ||||
|---|---|---|---|---|---|---|---|
| Total no. on BST Menu | Atypical | (%) | Total | Who Know the Meaning of the Symbol | (%) | ||
| Ward | |||||||
| Delivery room | 1700 | 1126 | 0 | 0.0% | 1 | 0 | 0.0% |
| General ward 1 | 36,750 | 31,168 | 170 | 0.5% | 2 | 0 | 0.0% |
| General ward 2 | 24,600 | 19,117 | 340 | 1.8% | 4 | 0 | 0.0% |
| General ward 3 | 44,600 | 35,553 | 399 | 1.1% | 5 | 3 | 60.0% |
| General ward 4 | 31,050 | 22,938 | 24 | 0.1% | 5 | 1 | 20.0% |
| General ward 5 | 21,150 | 16,531 | 43 | 0.3% | 2 | 0 | 0.0% |
| General ward 6 | 49,450 | 41,359 | 2139 | 5.2% | 2 | 2 | 100.0% |
| General ward 7 | 35,000 | 26,728 | 897 | 3.4% | 3 | 1 | 33.3% |
| General ward 8 | 25,550 | 20,578 | 80 | 0.4% | 2 | 0 | 0.0% |
| General ward 9 | 36,450 | 27,607 | 19 | 0.1% | 2 | 0 | 0.0% |
| General ward 10 | 22,100 | 17,255 | 18 | 0.1% | 1 | 0 | 0.0% |
| General ward 11 | 20,100 | 16,849 | 143 | 0.8% | 2 | 1 | 50.0% |
| General ward 12 | 10,000 | 3811 | 14 | 0.4% | 2 | 0 | 0.0% |
| General ward 13 | 28,350 | 20,667 | 7 | 0.0% | 2 | 0 | 0.0% |
| General ward 14 | 29,500 | 21,165 | 28 | 0.1% | 2 | 1 | 50.0% |
| General ward 15 | 26,200 | 21,115 | 7 | 0.0% | 4 | 0 | 0.0% |
| General ward 16 | 45,600 | 34,433 | 11 | 0.0% | 3 | 0 | 0.0% |
| General ward 17 | 28,750 | 19,990 | 23 | 0.1% | 6 | 0 | 0.0% |
| General ward 18 | 25,900 | 19,042 | 0 | 0.0% | 4 | 0 | 0.0% |
| General ward 19 | 19,800 | 15,082 | 71 | 0.5% | 2 | 0 | 0.0% |
| General ward 20 | 29,650 | 23,851 | 12 | 0.1% | 2 | 0 | 0.0% |
| ICU 1 | 43,450 | 41,547 | 27 | 0.1% | 7 | 1 | 14.3% |
| ICU 2 | 29,000 | 28,823 | 20 | 0.1% | 1 | 0 | 0.0% |
| ICU 3 | 37,750 | 37,086 | 47 | 0.1% | 2 | 0 | 0.0% |
| NICU | 6300 | 1831 | 0 | 0.0% | 4 | 0 | 0.0% |
| Neonates’ room | 5150 | 3522 | 29 | 0.8% | 1 | 0 | 0.0% |
| Outpatient clinic | |||||||
| Allergy and respiratory medicine | 150 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Cardiology | 1300 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Comprehensive check-up center | 100 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Endocrinology | 4950 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Family medicine | 150 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Gastroenterology | 150 | 0 | 0 | NA | 1 | 0 | 0.0% |
| General check-up center | 800 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Nephrology | 250 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Oncology and hematology | 50 | 2 | 0 | 0.0% | 1 | 0 | 0.0% |
| Pain medicine | 200 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Pediatrics | 50 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Special department | |||||||
| Bronchoscope | 200 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Cardiovascular center | 50 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Central injection | 2850 | 1787 | 0 | 0.0% | 1 | 0 | 0.0% |
| CT preparation | 250 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Day operation center | 1550 | 1202 | 0 | 0.0% | 1 | 0 | 0.0% |
| Dialysis | 25,500 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Emergency room | 28,300 | 262 | 0 | 0.0% | 1 | 0 | 0.0% |
| Nuclear medicine injection | 2100 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Nursing department | 250 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Operating room | 2350 | 0 | 0 | NA | 1 | 0 | 0.0% |
| Summary | 768,450 | 572,027 (13,786 patients) | 4568 | 0.8% | 94 | 10 | 10.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Choi, S.J.; Jeon, B.R.; Lee, Y.-W.; Oh, J.; Lee, Y.K. What We Should Consider in Point of Care Blood Glucose Test; Current Quality Management Status of a Single Institution. Medicina 2021, 57, 238. https://doi.org/10.3390/medicina57030238
Choi S, Choi SJ, Jeon BR, Lee Y-W, Oh J, Lee YK. What We Should Consider in Point of Care Blood Glucose Test; Current Quality Management Status of a Single Institution. Medicina. 2021; 57(3):238. https://doi.org/10.3390/medicina57030238
Chicago/Turabian StyleChoi, Sooin, Soo Jeong Choi, Byung Ryul Jeon, Yong-Wha Lee, Jongwon Oh, and You Kyoung Lee. 2021. "What We Should Consider in Point of Care Blood Glucose Test; Current Quality Management Status of a Single Institution" Medicina 57, no. 3: 238. https://doi.org/10.3390/medicina57030238
APA StyleChoi, S., Choi, S. J., Jeon, B. R., Lee, Y.-W., Oh, J., & Lee, Y. K. (2021). What We Should Consider in Point of Care Blood Glucose Test; Current Quality Management Status of a Single Institution. Medicina, 57(3), 238. https://doi.org/10.3390/medicina57030238






