An Update on Endodontic Microsurgery of Mandibular Molars: A Focused Review
Abstract
:1. Introduction
2. Anatomical Considerations
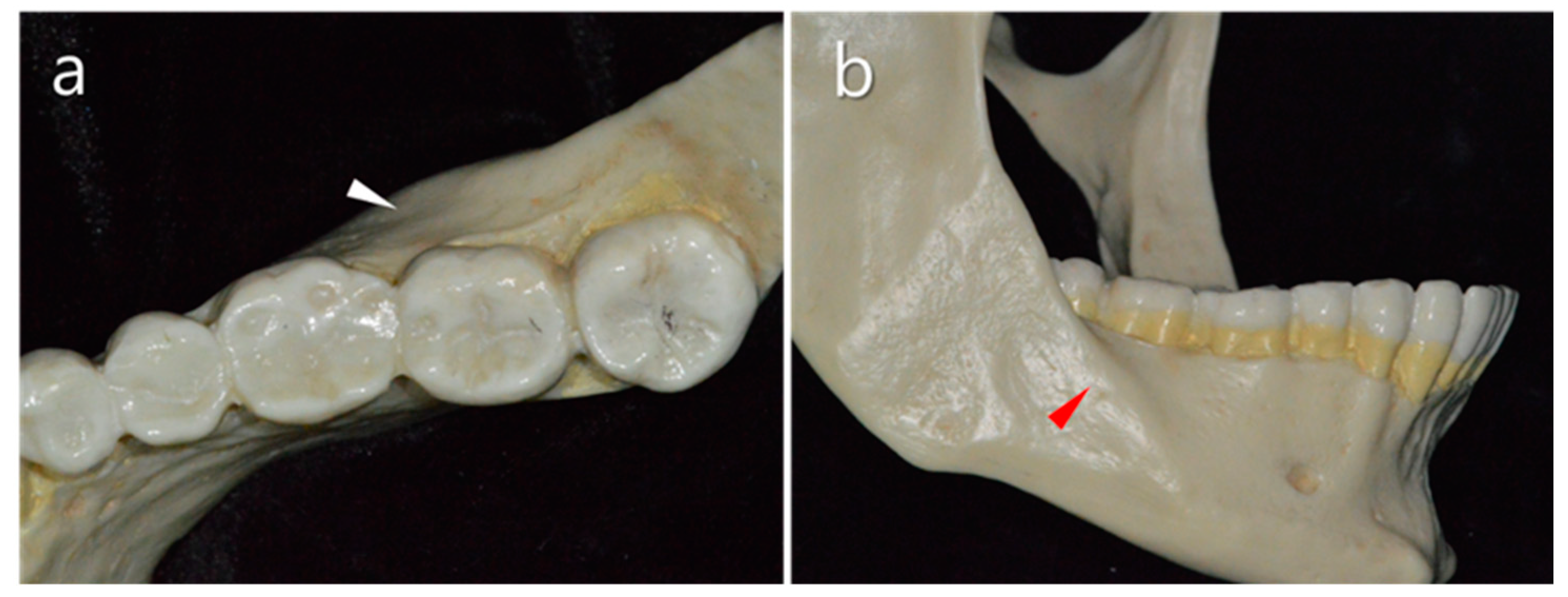
2.1. Thickness of the Buccal Bone
2.2. Vestibular Fornix
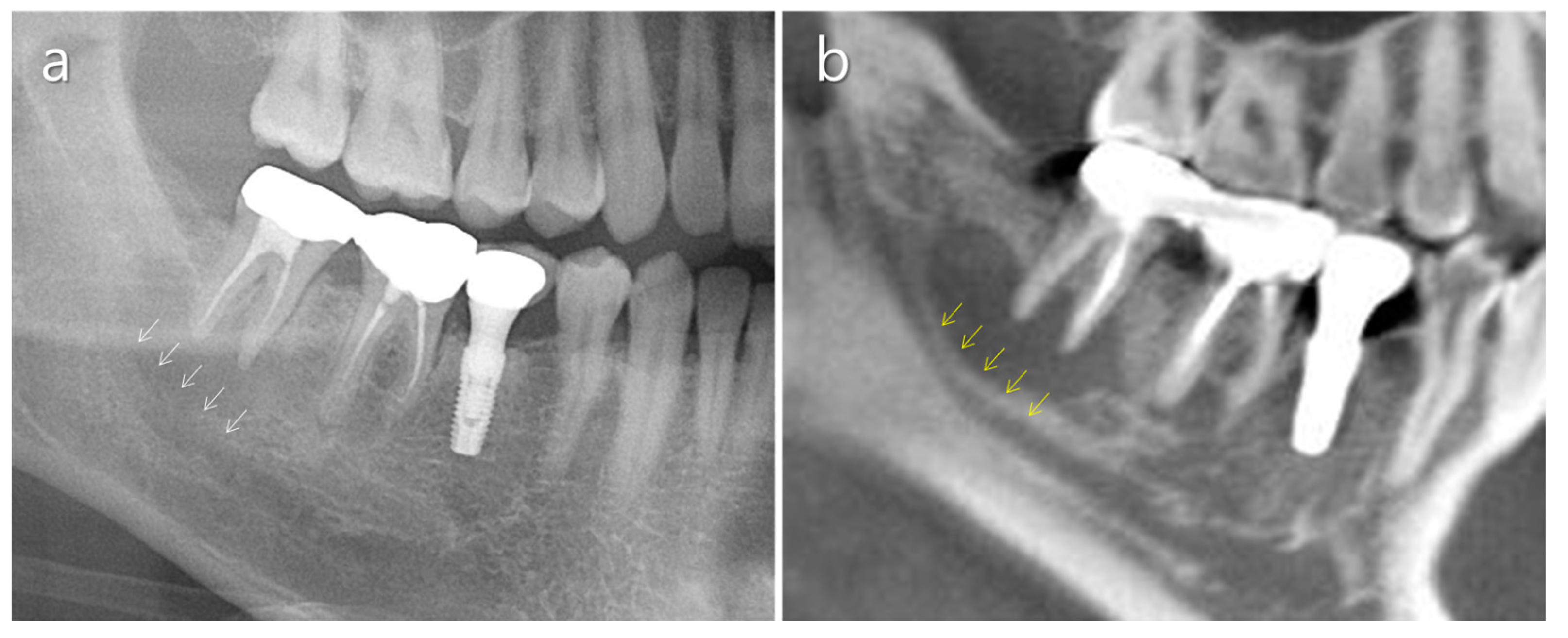
2.3. Mandibular Canal
2.4. Mental Nerve
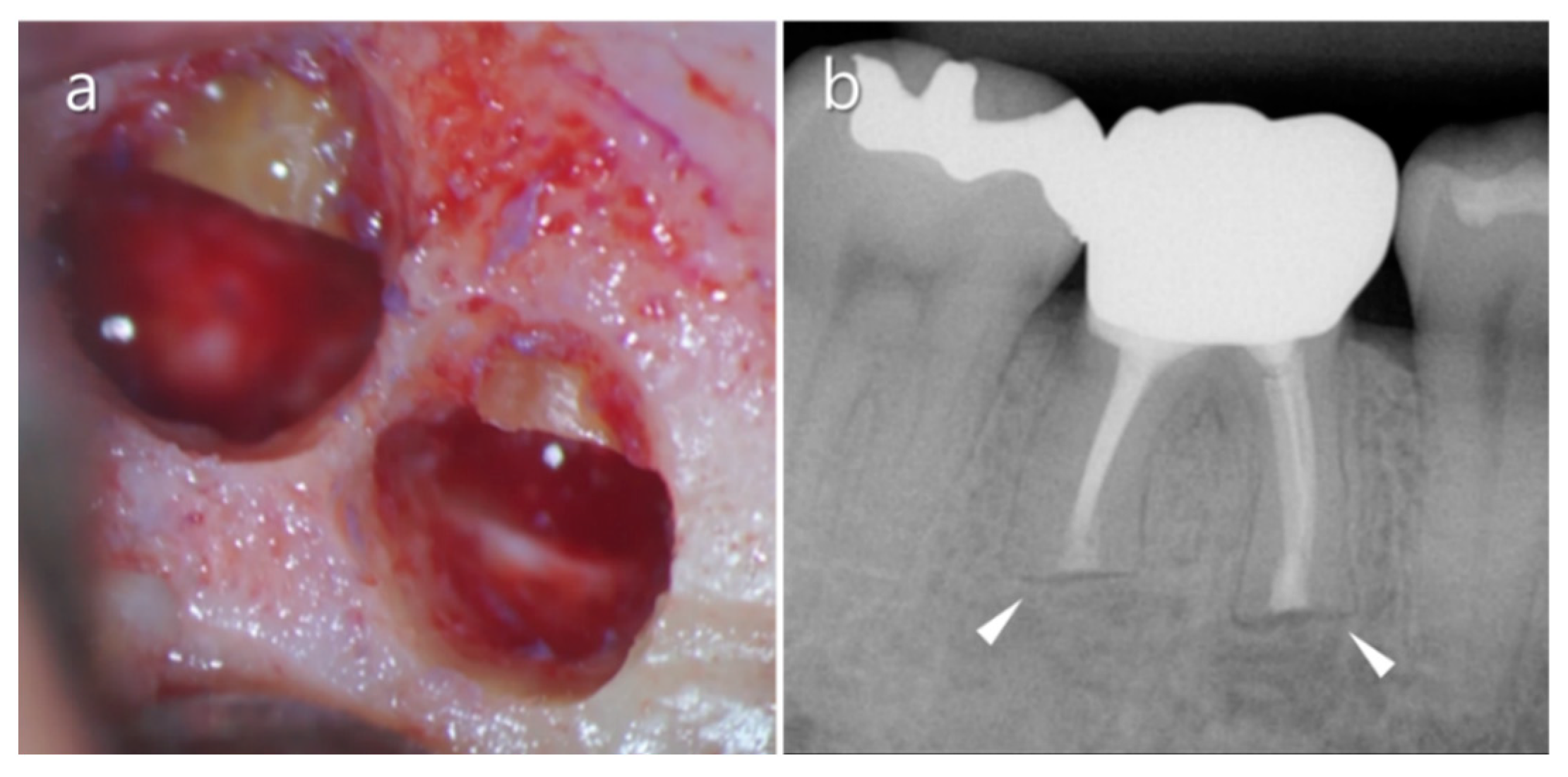
2.5. Distolingual Root
2.6. Mandibular Second Molars
3. Surgical Considerations
3.1. Flap Design
3.2. Osteotomy
3.3. Root-End Resection
3.4. Root-End Preparation
3.5. Root-End Filling
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tabassum, S.; Khan, F.R. Failure of endodontic treatment: The usual suspects. Eur. J. Dent. 2016, 10, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, L.; Jadun, S.; Darcey, J. Endodontic microsurgery. Part one: Diagnosis, patient selection and prognoses. Br. Dent. J. 2019, 226, 940–948. [Google Scholar] [CrossRef]
- Pallarés-Serrano, A.; Glera-Suarez, P.; Tarazona-Alvarz, B.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Prognostic factors after endodontic microsurgery: A retrospective Study of 111 cases with 5-9 years of follow-up. J. Endod. 2021, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, S.; Song, M.; Kang, D.R.; Kohli, M.R.; Kim, E. Outcome of endodontic micro-resurgery: A retrospective study based on propensity score-matched survival analysis. J. Endod. 2018, 44, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, K.; Wang, S.; Tiwari, S.K.; Ye, L.; Peng, L. Relationship between the mental foramen, mandibular canal, and the surgical access line of the mandibular posterior teeth: A cone-beam computed tomographic analysis. J. Endod. 2017, 43, 1262–1266. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, J.L.; Harroson, J.W. Posterior endodontic surgery: Anatomical considerations and clinical techniques. Int. Endod. J. 1985, 18, 8–34. [Google Scholar] [CrossRef]
- Bi, C.; Zhou, M.; Han, X.; Zhang, Y.; Zheng, P. Endodontic microsurgery with orthodontic treatment in a mandibular left molar with symptomatic apical periodontitis. J. Endod. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Kim, N.H.; Kim, S.; Karabucak, B.; Kim, E. Computer-aided design/computer-aided manufacturing-guided endodontic surgery: Guided osteotomy and apex localization in a mandibular molar with a thick buccal bone plate. J. Endod. 2018, 44, 665–670. [Google Scholar] [CrossRef]
- Kim, J.E.; Shim, J.S.; Shin, Y. A new minimally invasive guided endodontic microsurgery by cone beam computed tomography and 3-dimensional printing technology. Restor. Dent. Endod. 2019, 44, e29. [Google Scholar] [CrossRef]
- Gambarini, G.; Galli, M.; Stefanelli, L.V.; Di Nardo, D.; Morese, A.; Seracchiani, M.; De Angelis, F.; Di Carlo, S.; Testarelli, L. Endodontic microsurgery using dynamic avigation system: A case report. J. Endod. 2019, 45, 1397–1402. [Google Scholar] [CrossRef]
- Lin, L.; Skribner, J.; Shovlin, F.; Langeland, K. Periapical surgery of mandibular posterior teeth: Anatomical and surgical considerations. J. Endod. 1983, 9, 496–501. [Google Scholar] [CrossRef]
- Puciło, M.; Lipski, M.; Sroczyk-Jaszczyńska, M.; Puciło, A.; Nowicka, A. The anatomical relationship between the roots of erupted permanent teeth and the mandibular canal: A systematic review. Surg. Radiol. Anat. 2020, 42, 529–542. [Google Scholar] [CrossRef]
- Aksoy, U.; Aksoy, S.; Orhan, K. A cone-beam computed tomography study of the anatomical relationships between mandibular teeth and the mandibular canal, with a review of the current literature. Microsc. Res. Tech. 2018, 81, 308–314. [Google Scholar] [CrossRef]
- Kawashima, Y.; Sakai, O.; Shosho, D.; Kaneda, T.; Gohel, A. Proximity of the mandibular canal to teeth and cortical bone. J. Endod. 2016, 42, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Velvart, P.; Hecker, H.; Tillinger, G. Detection of the apical lesion and the mandibular canal in conventional radiography and computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Peñarrocha, M.; García, B.; Martínez, J.M.; Gay-Escoda, C. Distance between periapical lesion and mandibular canal as a factor in periapical surgery in mandibular molars. J. Oral Maxillofac. Surg. 2008, 66, 2461–2466. [Google Scholar] [CrossRef]
- Igarashi, C.; Kobayashi, K.; Yamamoto, A.; Morita, Y.; Tanaka, M. Double mental foramina of the mandible on computed tomography images: A case report. Oral Radiol. 2004, 20, 68–71. [Google Scholar] [CrossRef]
- Cagirankaya, L.B.; Kansu, H. An accesory mental foramen: A case report. J. Contemp. Dent. Pract. 2008, 9, 98–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieser, J.; Kuzmanovic, D.; Payne, A.; Dennison, J.; Herbison, P. Patterns of emergence of the human mental nerve. Arch. Oral Biol. 2002, 47, 743–747. [Google Scholar] [CrossRef]
- Han, S.S.; Hwang, J.J.; Jeong, H.G. Accessory mental foramina associated with neurovascular bundle in Korean population. Surg. Radiol. Anat. 2016, 38, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, J.; Watanabe, K.; Saga, T.; Kikuta, S.; Tabira, Y.; Kitashima, S.; Fisahn, C.; Alonso, F.; Tubbs, R.S.; Kusukawa, J.; et al. Undetected small accessory mental foramina using cone-beam computed tomography. Cureus 2017, 9, e1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwanaga, J.; Saga, T.; Tabira, Y.; Nakamura, M.; Kitashima, S.; Watanabe, K.; Kusukawa, J.; Yamaki, K.I. The clinical anatomy of accessory mental nerves and foramina. Clin. Anat. 2015, 28, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, M.; Rankow, H.J. Accessory branch of the mental nerve. J. Endod. 2000, 26, 619–620. [Google Scholar] [CrossRef]
- Kim, S.; Kratchman, S. Modern endodontic surgery concepts and practice: A review. J. Endod. 2006, 32, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Q.H.; Zhou, X.D.; Tang, L.; Wang, Q.; Zheng, G.N.; Huang, D.M. Evaluation of the root and canal morphology of mandibular first permanent molars in a western Chinese population by cone-beam computed tomography. J. Endod. 2010, 36, 1786–1789. [Google Scholar] [CrossRef]
- Tu, M.G.; Tsai, C.C.; Jou, M.J.; Chen, W.L.; Chang, Y.F.; Chen, S.Y.; Cheng, H.W. Prevalence of three-rooted mandibular first molars among Taiwanese individuals. J. Endod. 2007, 33, 1163–1166. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Choi, H.J.; Jung, I.Y.; Jung, H.S.; Kim, S.O. The prevalence and morphologic classification of distolingual roots in the mandibular molars in a Korean population. J. Endod. 2010, 36, 653–657. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yang, S.E. Cone-beam computed tomography study of incidence of distolingual root and distance from distolingual canal to buccal cortical bone of mandibular first molars in a Korean population. J. Endod. 2012, 38, 301–304. [Google Scholar] [CrossRef]
- Wang, H.G.; Xu, N.; Yu, Q. Endodontic microsurgical treatment of a three-rooted mandibular first molar with separate distolingual root: Report of one case. Chin. J. Dent. Res. 2016, 19, 171–174. [Google Scholar]
- Littner, M.M.; Kaffe, I.; Tamise, A.; Dicapua, P. Relationship between the apices of the lower molars and mandibular canals—A radiographic study. Oral Surg. Oral Med. Oral Pathol. 1986, 62, 595–602. [Google Scholar] [CrossRef]
- Song, Y. Periapical surgery of the mandibular 2nd molar: Case report. J. Kor. Acad. Endod. 2012, 13, 41–45. [Google Scholar]
- Velvart, P. Papilla base incision: A new approach to recession-free healing of the interdental papilla after endodontic surgery. Int. Endod. J. 2002, 35, 453–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velvart, P.; Ebner-Zimmermann, U.; Ebner, J.P. Comparison of long-term papilla healing following sulcular full thickness flap and papilla base flap in endodontic surgery. Int. Endod. J. 2004, 37, 687–693. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T.; Vinzens-Majaniemi, T.; Bürgin, W.; Jensen, S. Changes of periodontal parameters following apical surgery: A prospective clinical study of three incision techniques. Int. Endod. J. 2007, 40, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.R.; Fayad, M.I.; Witherspoon, D.E. Periradicular Surgery. In Cohen’s Pathways of the Pulp, 10th ed.; Hargreaves, K.M., Cohen, S., Eds.; Mosby: St. Louis, MO, USA, 2011; pp. 720–776. [Google Scholar]
- Ingle, J.I.; Beveridge, E.E.; Cummings, R.; Frank, A.X.; Glick, D.H.; Wolfson, B. Endodontic surgery. In Endodontics, 2nd ed.; Ingle, J.I., Beveridge, E.E., Eds.; Lea & Febiger: Philadelphia, PA, USA, 1976; pp. 594–684. [Google Scholar]
- Kim, S.; Pecora, G.; Rubinstein, R. Comparison of traditional and microsurgery in endodontics. In Color Atlas of Microsurgery in Endodontics; Kim, S., Pecora, G., Rubinstein, R., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2001; pp. 5–11. [Google Scholar]
- Moiseiwitsch, J.R. Avoiding the mental foramen during periapical surgery. J. Endod. 1995, 21, 340–342. [Google Scholar] [CrossRef]
- Rubinstein, R.A.; Kim, S. Short-term observation of the results of endodontic surgery with the use of a surgical operation microscope and Super-EBA as root-end filling material. J. Endod. 1999, 25, 43–48. [Google Scholar] [CrossRef]
- Floratos, S.; Kim, S. Modern endodontic microsurgery concepts: A clinical update. Dent. Clin. N. Am. 2017, 61, 81–91. [Google Scholar] [CrossRef]
- Carcía-Mira, B.; Ortega-Sánchez, B.; Peñarrocha-Diago, M.; Peñarrocha-Diago, M. Ostectomy versus osteotomy with repositioning of the vestibular cortical in periapical surgery of mandibular molars: A preliminary study. Med. Oral Patol. Oral Cir. Bucal. 2010, 15, e628–e632. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Yu, Y.H.; Wang, Y.; Kim, E.; Kim, S. The application of “bone window” technique in endodontic microsurgery. J. Endod. 2020, 46, 872–880. [Google Scholar] [CrossRef]
- Kim, U.; Kim, S.; Kim, E. The application of “bone window technique” using piezoelectric saws and a CAD/CAM-guided surgical stent in endodontic microsurgery on a mandibular molar case. Restor. Dent. Endod. 2020, 45, e27. [Google Scholar] [CrossRef]
- Kulakov, A.A.; Badalyan, V.A.; Stepanyan, Z.M. Increasing the effectiveness of mandibular molars root resection surgery using retrograde endodontic revision. Stomatologiia 2018, 97, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Karunakaran, J.V.; PremKumar, M.M.; Aarthi, G.; Jayaprakash, N.; Kumar, S.S. Isthmus incidence in human permanent mandibular first molars of a south Indian population: A cone-beam computerized tomographic study. J. Pharm. Bioallied. Sci. 2019, 11, S468–S473. [Google Scholar] [CrossRef]
- von Arx, T. Frequency and type of canal isthmuses in first molars detected by endoscopic inspection during periradicular surgery. Int. Endod. J. 2005, 38, 160–168. [Google Scholar] [CrossRef]
- Kang, S.; Yu, H.W.; Shin, Y.; Karabucak, B.; Kim, S.; Kim, E. Topographic analysis of the isthmus in mesiobuccal and mesial roots of first molars in a south Korean population. Sci. Rep. 2020, 10, 1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Jung, H.; Kim, S.; Shin, S.J.; Kim, E. The Influence of an isthmus on the outcomes of surgically treated molars: A retrospective study. J. Endod. 2016, 42, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.I.; Peters, O.A.; Barbakow, F. An in vitro study comparing root-end cavities prepared by diamond coated and stainless steel ultrasonic retrotips. Int. Endod. J. 2001, 34, 142–148. [Google Scholar] [CrossRef]
- Gondim, E., Jr.; Figueiredo Almeida de Gomes, B.P.; Ferraz, C.C.; Teixeira, F.B.; de Souza-Filho, F.J. Effect of sonic and ultrasonic retrograde cavity preparation on the integrity of root apices of freshly extracted human teeth: Scanning electron microscopy analysis. J. Endod. 2002, 28, 646–650. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.; Jia, L.; Wang, Y.; Liu, W.; Zhou, X.; Johnson, T.M.; Huang, D. Materials for retrograde filling in root canal therapy. Cochrane Database Syst. Rev. 2016, 12, CD005517. [Google Scholar] [CrossRef] [PubMed]
- Dorn, S.O.; Gartner, A.H. Retrograde filling materials: A retrospective success-failure study of amalgam, EBA, and IRM. J. Endod. 1990, 16, 391–393. [Google Scholar] [CrossRef]
- Solanki, N.P.; Venkappa, K.K.; Shah, N.C. Biocompatibility and sealing ability of mineral trioxide aggregate and biodentine as root-end filling material: A systematic review. J. Conserv. Dent. 2018, 21, 10–15. [Google Scholar]
- Shokouhinejad, N.; Jafargholizadeh, L.; Khoshkhounejad, M.; Nekoofar, M.H.; Raoof, M. Surface microhardness of three thicknesses of mineral trioxide aggregate in different setting conditions. Restor. Dent. Endod. 2014, 39, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galarça, A.D.; Da Rosa, W.L.; Da Silva, T.M.; da Silveira Lima, G.; Carreño, N.L.; Pereira, T.M.; Aguirre Guedes, O.; Borges, A.H.; da Silva, A.F.; Piva, E. Physical and biological properties of a high-plasticity tricalcium silicate cement. BioMed Res. Int. 2018, 2018, 8063262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellucci, A.; Papaleoni, M. Il MAP (Micro Apical Placement) System: Un perfetto carrier per MTA in endodonzia clinica e chirurgica. Inf. Endod. 2009, 12, 14–25. [Google Scholar]
- Malkondu, Ö.; Karapinar Kazandağ, M.; Kazazoğlu, E. A review on biodentine, a contemporary dentine replacement and repair material. BioMed Res. Int. 2014, 2014, 160951. [Google Scholar] [CrossRef] [Green Version]

















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.M.; Kim, E.; Min, K.-S. An Update on Endodontic Microsurgery of Mandibular Molars: A Focused Review. Medicina 2021, 57, 270. https://doi.org/10.3390/medicina57030270
Jang SM, Kim E, Min K-S. An Update on Endodontic Microsurgery of Mandibular Molars: A Focused Review. Medicina. 2021; 57(3):270. https://doi.org/10.3390/medicina57030270
Chicago/Turabian StyleJang, Sun Mi, Euiseong Kim, and Kyung-San Min. 2021. "An Update on Endodontic Microsurgery of Mandibular Molars: A Focused Review" Medicina 57, no. 3: 270. https://doi.org/10.3390/medicina57030270
APA StyleJang, S. M., Kim, E., & Min, K.-S. (2021). An Update on Endodontic Microsurgery of Mandibular Molars: A Focused Review. Medicina, 57(3), 270. https://doi.org/10.3390/medicina57030270






