Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update
Abstract
:1. Introduction
2. Materials & Methods
3. Results
3.1. Causative Pathogens
3.2. General Therapeutic Approaches
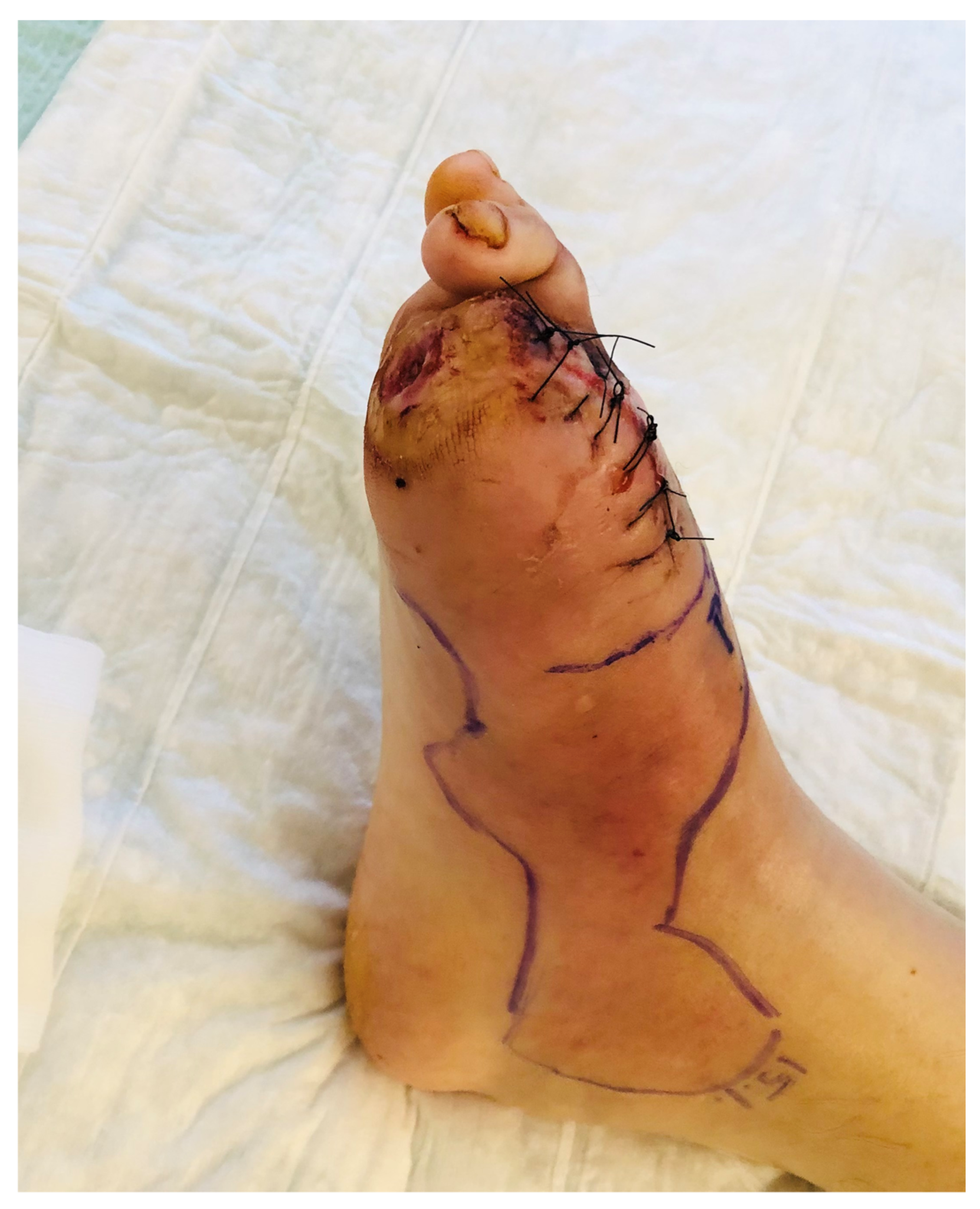
3.3. Surgical Treatment
3.3.1. Surgery for the Prevention of Future DFO Episodes
3.3.2. Surgical Amputations
3.3.3. Surgical Reconstruction
3.4. Systemic Antibiotics
3.4.1. Antibiotic Stewardship in DFO
3.4.2. Route of Antibiotic Administration
3.4.3. The Potential Role of Rifampin in DFO
3.4.4. Duration of Antibiotic Therapy
3.4.5. Antibiotic Therapy after Amputation for Residual Infection
3.4.6. Intra-Osseus Local Antimicrobials
3.4.7. Clinical Pathways, Antibiotic Stewardship and Multimodal Interventions
3.5. Outcomes of Therapies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAD | Peripheral arterial disease |
| WBC | White blood cell |
| MRI | Magnetic resonance imaging |
| PET/CT | Positron emission tomography/computed tomography |
References
- Lipsky, B.A.; Van Asten, S.A.V. An Evidence-Based Approach to Treating Diabetic Foot Osteomyelitis. In Functional Limb Salvage: The Multidisciplinary Approach; Christopher, E., Attinger, J., Steinberg, S., Eds.; Springer: New York, NY, USA, 2021; Chapter 14, Book # 48329, in press. [Google Scholar]
- Uçkay, I.; Aragón-Sánchez, J.; Lew, D.; Lipsky, B.A. Diabetic foot infections: What have we learned in the last 30 years? Int. J. Infect. Dis. 2015, 40, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uçkay, I.; Gariani, K.; Dubois-Ferrière, V.; Suvà, D.; Lipsky, B.A. Diabetic foot infections: Recent literature and cornerstones of management. Curr. Opin. Infect. Dis. 2016, 29, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ertuğrul, B.; Uçkay, I.; Schöni, M.; Peter-Riesch, B.; Lipsky, B.A. Management of diabetic foot infections in the light of recent literature and new international guidelines. Expert Rev. Anti-Infect. Ther. 2020, 18, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Yammine, K.; Hayek, F.; Assi, C. A meta-analysis of mortality after minor amputation among patients with diabetes and/or peripheral vascular disease. J. Vasc. Surg. 2020, 72, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Glaudemans, A.W.J.M.; Uckay, I.; Lipsky, B.A. Challenges in diagnosing infection in the diabetic foot. Diabet. Med. 2015, 32, 748–759. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.G.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef] [Green Version]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Peters, E.J.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, 3280. [Google Scholar] [CrossRef] [Green Version]
- Uçkay, I.; Pires, D.; Agostinho, A.; Guanziroli, N.; Öztürk, M.; Bartolone, P.; Tscholl, P.; Betz, M.; Pittet, D. Enterococci in orthopaedic infections: Who is at risk getting infected? J. Infect. 2017, 75, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Jamei, O.; Gjoni, S.; Zenelaj, B.; Kressmann, B.; Belaieff, W.; Hannouche, D.; Uçkay, I. Which Orthopaedic Patients Are Infected with Gram-negative Non-fermenting Rods? J. Bone Jt. Infect. 2017, 2, 73–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charles, P.G.; Uçkay, I.; Kressmann, B.; Emonet, S.; Lipsky, B.A. The role of anaerobes in diabetic foot infections. Anaerobe 2015, 34, 8–13. [Google Scholar] [CrossRef]
- Al-Mayahi, M.; Cian, A.; Lipsky, B.A.; Suvà, D.; Müller, C.; Landelle, C.; Miozzari, H.H.; Uçkay, I. Administration of antibiotic agents before intraoperative sampling in orthopedic infections alters culture results. J. Infect. 2015, 71, 518–525. [Google Scholar] [CrossRef] [Green Version]
- Wuarin, L.; Abbas, M.; Harbarth, S.; Waibel, F.; Holy, D.; Burkhard, J.; Uçkay, I. Changing perioperative prophylaxis during antibiotic therapy and iterative debridement for orthopedic infections? PLoS ONE 2019, 14, e0226674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenelaj, B.; Bouvet, C.; Lipsky, B.A.; Uçkay, I. Do Diabetic Foot Infections with Methicillin-Resistant Staphylococcus aureus Differ from Those With Other Pathogens? Int. J. Low. Extrem. Wounds 2014, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Senneville, E.; Joulie, D.; Blondiaux, N.; Robineau, O. Surgical techniques for bone biopsy in diabetic foot infection, and association between results and treatment duration. J. Bone Jt. Infect. 2020, 5, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Couturier, A.; Chabaud, A.; Desbiez, F.; Descamps, S.; Petrosyan, E.; Letertre-Gilbert, P.; Mrozek, N.; Vidal, M.; Tauveron, I.; Maqdasy, S.; et al. Comparison of microbiological results obtained from per-wound bone biopsies versus transcutaneous bone biopsies in diabetic foot osteomyelitis: A prospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1287–1291. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Lipsky, B.A. Systemic Antibiotic Therapy for Chronic Osteomyelitis in Adults. Clin. Infect. Dis. 2012, 54, 393–407. [Google Scholar] [CrossRef] [Green Version]
- Jeffcoate, W.J.; Lipsky, B.A. Controversies in Diagnosing and Managing Osteomyelitis of the Foot in Diabetes. Clin. Infect. Dis. 2004, 39, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Lázaro-Martínez, J.L.; Aragón-Sánchez, J.; García-Morales, E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: A randomized comparative trial. Diabetes Care 2014, 37, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Martínez, J.L.L.; Álvarez, Y.G.; Tardáguila-García, A.; Morales, E.G. Optimal management of diabetic foot osteomyelitis: Challenges and solutions. Diabetes Metab. Syndr. Obes. 2019, 12, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Aicale, R.; Cipollaro, L.; Esposito, S.; Maffulli, N. An evidence based narrative review on treatment of diabetic foot osteomyelitis. Surgeon 2020, 18, 311–320. [Google Scholar] [CrossRef]
- Aragón-Sánchez, J.; Lázaro-Martínez, J.; Alvaro-Afonso, F.J.; Molinés-Barroso, R. Conservative surgery of diabetic forefoot osteomyelitis: How can I operate on this patient without amputation? Int. J. Low. Extrem. Wounds 2015, 14, 108–131. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Sánchez, J. Clinical-pathological characterization of diabetic foot infections: Grading the severity of osteomyelitis. Int. J. Low. Extrem. Wounds 2012, 11, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Aragón-Sánchez, J.; Lázaro-Martínez, J.; Hernández-Herrero, M.J.; Campillo-Vilorio, N.; Quintana-Marrero, Y.; García-Morales, E. Does osteomyelitis in the feet of patients with diabetes really recur after surgical treatment? Natural history of a surgical series. Diabetes Med. 2012, 29, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Lázaro-Martínez, J.L.; García-Madrid, M.; García-Álvarez, Y.; Álvaro-Afonso, F.J.; Sanz-Corbalán, I.; García-Morales, E. Conservative surgery for chronic diabetic foot osteomyelitis: Procedures and recommendations. J. Clin. Orthop. Trauma 2021, 16, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Biz, C.; Ruggieri, P. Minimally invasive surgery: Osteotomies for diabetic foot disease. Foot Ankle Clin. 2020, 25, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Cychosz, C.C.; Phisitkul, P.; Belatti, D.A.; Wukich, D. Current concepts review: Preventive and therapeutic strategies for diabetic foot ulcers. Foot Ankle Int. 2015, 11, 1–10. [Google Scholar]
- Rasmussen, A.; Bjerre-Christensen, U.; Almdal, T.P.; Holstein, P. Percutaneous flexor tenotomy for preventing and treating toe ulcers in people with diabetes mellitus. J. Tissue Viability 2013, 2, 68–73. [Google Scholar] [CrossRef]
- Nieto-García, E.; Ferrer-Torregrosa, J.; Ramírez-Andrés, L.; Nieto-González, E.; Martinez-Nova, A.; Barrios, C. The impact of associated tenotomies on the outcome of incomplete phalangeal osteotomies for lesser toe deformities. J. Orthop. Surg. Res. 2019, 14, 308. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Hwang, S.; Lee, Y. Selective Plantar Fascia Release for Nonhealing Diabetic Plantar Ulcerations. J. Bone Jt. Surg. 2012, 94, 1297–1302. [Google Scholar] [CrossRef]
- Gariani, K.; Lebowitz, D.; Von Dach, E.; Kressmann, B.; Lipsky, B.A.; Uçkay, I. Remission in diabetic foot infections: Duration of antibiotic therapy and other possible associated factors. Diabetes Obes. Metab. 2019, 21, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Lacraz, A.; Armand, S.; Turcot, K.; Carmona, G.; Stern, R.; Borens, O.; Assal, M. Comparison of the Otto Bock solid ankle cushion heel foot with wooden keel to the low-cost CR-Equipements™ solid ankle cushion heel foot with polypropylene keel: A randomized prospective double-blind crossover study assessing patient satisfaction and energy expenditure. Prosthet. Orthot. Int. 2017, 41, 258–265. [Google Scholar] [PubMed]
- Carmona, G.A.; Lacraz, A.; Hoffmeyer, P.; Assal, M. Incidence of major lower limb amputation in Geneva: Twenty-one years of observation. Rev. Med. Suisse 2014, 10, 1997–1998. [Google Scholar] [PubMed]
- Zingg, M.; Lacraz, A.; Robert-Ebadi, H.; Waibel, F.; Berli, M.; Uçkay, I. Transcutaneous oxygen pressure values often fail to predict stump failures after foot or limb amputation in chronically ischemic patients. Clin. Surg. 2019, 1–6. [Google Scholar] [CrossRef]
- Waibel, F.W.A.; Klammer, A.; Götschi, T.; Uçkay, I.; Böni, T.; Berli, M.C. Outcome After Surgical Treatment of Calcaneal Osteomyelitis. Foot Ankle Int. 2019, 40, 562–567. [Google Scholar] [CrossRef]
- Waibel, F.W.A.; Uçkay, I.; Sairanen, K.; Waibel, L.; Berli, M.C.; Böni, T.; Gariani, K.; Lipsky, B.A. Diabetic calcaneal osteomyelitis. Infez. Med. 2019, 27, 225–238. [Google Scholar] [PubMed]
- Walsh, T.P.; Yates, B.J. Calcanectomy: Avoiding major amputation in the presence of calcaneal osteomyelitis—A case series. Foot 2013, 23, 130–135. [Google Scholar] [CrossRef]
- Gariani, K.; A Waibel, F.W.; Viehöfer, A.F.; Uçkay, I. Plantar Fasciitis in Diabetic Foot Patients: Risk Factors, Pathophysiology, Diagnosis, and Management. Diabetes Metab. Syndr. Obes. 2020, 13, 1271–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Chin, K.M.; Lee, S.; Yap, H.Y.; Ch’Ng, J.K.; Chng, S.P.; Tay, H.T. Maximally Disfiguring Surgery for Forefoot Osteomyelitis: Time for a Rethink? Int. J. Low. Extrem. Wounds 2020, 19, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald O’Connor, E.J.; Vesely, M.; Holt, P.J.; Jones, K.G.; Thompson, M.M.; Hinchliffe, R.J. A systematic review of free tissue transfer in the management of non-traumatic lower extremity wounds in patients with diabetes. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Uçkay, I.; Lipsky, B.A. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin. Pharmacother. 2015, 16, 821–832. [Google Scholar] [CrossRef]
- Gariani, K.; Pham, T.-T.; Kressmann, B.; Jornayvaz, F.R.; Gastaldi, G.; Stafylakis, D.; Philippe, J.; Lipsky, B.A.; Uçkay, I. Three versus six weeks of antibiotic therapy for diabetic foot osteomyelitis: A prospective, randomized, non-inferiority pilot trial. Clin. Infect. Dis. 2020, 1758. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Kressmann, B.; Malacarne, S.; Toumanova, A.; Jaafar, J.; Lew, D.; Lipsky, B.A. A randomized, controlled study to investigate the efficacy and safety of a topical gentamicin-collagen sponge in combination with systemic antibiotic therapy in diabetic patients with a moderate or severe foot ulcer infection. BMC Infect. Dis. 2018, 18, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senneville, E.; Robineau, O. Treatment options for diabetic foot osteomyelitis. Expert Opin. Pharmacother. 2017, 18, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-K.; Rombach, I.; Zambellas, R.; Walker, S.A.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Lebowitz, D.; Kressmann, B.; Von Dach, E.; Sendi, P.; Waibel, F.; Berli, M.; Huber, T.; Lipsky, B.A.; Uçkay, I. Oral amoxicillin-clavulanate for treating diabetic foot infections. Diabetes Obes. Metab. 2019, 21, 1483–1486. [Google Scholar] [CrossRef]
- Wilson, B.M.; Bessesen, M.T.; Doros, G.; Brown, S.T.; Saade, E.; Hermos, J.; Perez, F.; Skalweit, M.; Spellberg, B.; Bonomo, R.A. Adjunctive Rifampin Therapy For Diabetic Foot Osteomyelitis in the Veterans Health Administration. JAMA Netw. Open 2019, 2, 1916003. [Google Scholar] [CrossRef] [PubMed]
- Bessesen, M.T.; Doros, G.; Henrie, A.M.; Harrington, K.M.; Hermos, J.A.; Bonomo, R.A.; Ferguson, R.E.; Huang, G.D.; Brown, S.T. A multicenter randomized placebo controlled trial of rifampin to reduce pedal amputations for osteomyelitis in veterans with diabetes (VA INTREPID). BMC Infect. Dis. 2020, 20, 23. [Google Scholar] [CrossRef]
- Peters, E.J.G.; Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; et al. Interventions in the management of infection in the foot in diabetes: A systematic review. Diabetes Metab. Res. Rev. 2020, 36, e3282. [Google Scholar] [CrossRef] [Green Version]
- Bharati, S.P.; Sukumaran, S.K. A review on the current principles of antibiotic therapy for diabetic foot infection. Infect. Disord. Drug Targets 2020. [Google Scholar] [CrossRef]
- Van Asten, S.A.V.; Mithani, M.; Peters, E.J.G.; La Fontaine, J.; Kim, P.J.; Lavery, L.A. Complications during treatment of diabetic foot osteomyelitis. Diabetes Res. Clin. Pract. 2018, 135, 58–64. [Google Scholar] [CrossRef]
- Tone, A.; Nguyen, S.; Devemy, F.; Topolinski, H.; Valette, M.; Cazaubiel, M.; Fayard, A.; Beltrand, É.; Lemaire, C.; Senneville, É. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic toot osteomyelitis: A multicenter open-label controlled randomized study. Diabetes Care 2015, 38, 302–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waibel, F.; Berli, M.; Catanzaro, S.; Sairanen, K.; Schöni, M.; Böni, T.; Burkhard, J.; Holy, D.; Huber, T.; Bertram, M.; et al. Optimization of the antibiotic management of diabetic foot infections: Protocol for two randomized controlled trials. Trials 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, T.-T.; Wetzel, O.; Gariani, K.; Kressmann, B.; Jornayvaz, F.R.; Lipsky, B.A.; Uçkay, I. Is routine measurement of the serum C-reactive protein level helpful during antibiotic therapy for diabetic foot infection? Diabetes Obes. Metab. 2021, 23, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Fritz, B.G.; Vickery, K.; Schwarzer, S.; Sharma, V.; Biggs, N.; Radzieta, M.; Jeffries, T.T.; Dickson, H.G.; Jensen, S.O.; et al. Analysis of proximal bone margins in diabetic foot osteomyelitis by conventional culture, DNA sequencing and microscopy. APMIS 2019, 127, 660–670. [Google Scholar] [CrossRef]
- Rossel, A.; Lebowitz, D.; Gariani, K.; Abbas, M.; Kressmann, B.; Assal, M.; Tscholl, P.; Stafylakis, D.; Uçkay, I. Stopping antibiotics after surgical amputation in diabetic foot and ankle infections—A daily practice cohort. Endocrinol. Diabetes Metab. 2019, 2, e00059. [Google Scholar] [CrossRef]
- Kowalski, T.J.; Matsuda, M.; Sorenson, M.D.; Gundrum, J.D.; Agger, W.A. The Effect of Residual Osteomyelitis at the Resection Margin in Patients with Surgically Treated Diabetic Foot Infection. J. Foot Ankle Surg. 2011, 50, 171–175. [Google Scholar] [CrossRef]
- Atway, S.; Nerone, V.S.; Springer, K.D.; Woodruff, D.M. Rate of Residual Osteomyelitis after Partial Foot Amputation in Diabetic Patients: A Standardized Method for Evaluating Bone Margins with Intraoperative Culture. J. Foot Ankle Surg. 2012, 51, 749–752. [Google Scholar] [CrossRef]
- Saltoğlu, N.; Yemisen, M.; Ergonul, O.; Kadanali, A.; Karagoz, G.; Batirel, A.; Ak, O.; Eraksoy, H.; Cagatay, A.; Vatan, A.; et al. Predictors for limb loss among patient with diabetic foot infections: An observational retrospective multicentric study in Turkey. Clin. Microbiol. Infect. 2015, 21, 659–664. [Google Scholar] [CrossRef] [Green Version]
- Mijuskovic, B.; Kuehl, R.; Widmer, A.; Jundt, G.; Frei, R.; Gürke, L.; Wolff, T. Culture of Bone Biopsy Specimens Overestimates Rate of Residual Osteomyelitis After Toe or Forefoot Amputation. J. Bone Jt. Surg. 2018, 100, 1448–1454. [Google Scholar] [CrossRef]
- Panagopoulos, P.; Drosos, G.; Maltezos, E.; Papanas, N. Local antibiotic delivery systems in diabetic foot osteomyelitis: Time for one step beyond? Int. J. Low. Extrem. Wounds 2015, 14, 87–91. [Google Scholar] [CrossRef]
- Van Vugt, T.A.G.; Arts, J.J.; Geurts, J.A.P. Antibiotic-loaded polymethylmethacrylate beads and spacers in treatment of orthopedic infections and the role of biofilm formation. Front. Microbiol. 2019, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Hake, M.E.; Young, H.; Hak, D.J.; Stahel, P.F.; Hammerberg, E.M.; Mauffrey, C. Local antibiotic therapy strategies in orthopaedic trauma: Practical tips and tricks and review of the literature. Injury 2015, 46, 1447–1456. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.-H.; Zhou, C.-H.; Song, H.-J.; Cheng, G.-Y.; Zhang, H.-A.; Fang, J.; Tao, R. Infected bone resection plus adjuvant antibiotic-impregnated calcium sulfate versus infected bone resection alone in the treatment of diabetic forefoot osteomyelitis. BMC Musculoskelet. Disord. 2019, 20, 246. [Google Scholar] [CrossRef] [Green Version]
- Uçkay, I.; Berli, M.; Sendi, P.; Lipsky, B.A. Principles and practice of antibiotic stewardship in the management of diabetic foot infections. Curr. Opin. Infect. Dis. 2019, 32, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Hassan-Reshat, S.; Newsholme, W. Retrospective analysis of diabetic foot osteomyelitis management and outcome in a tertiary care hospital in the UK. PLoS ONE 2019, 14, e0216701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aragón-Sánchez, J.; Víquez-Molina, G.; López-Valverde, M.E.; Rojas-Bonilla, J.M.; Murillo-Vargas, C. Surgical Diabetic Foot Infections: Is Osteomyelitis Associated With a Worse Prognosis? Int. J. Low. Extrem. Wounds 2021, 2. [Google Scholar] [CrossRef]
- Lebowitz, D.; Gariani, K.; Kressmann, B.; von Dach, E.; Huttner, B.; Bartolone, P.; Lê, N.; Mohamad, M.; Lipsky, B.A.; Uçkay, I. Are antibiotic-resistant pathogens more common in subsequent episodes of diabetic foot infection? Int. J. Infect. Dis. 2017, 59, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Häller, T.V.; Kaiser, P.; Kaiser, D.; Berli, M.C.; Uçkay, I.; Waibel, F.W. Outcome of Ray Resection as Definitive Treatment in Forefoot Infection or Ischemia: A Cohort Study. J. Foot Ankle Surg. 2020, 59, 27–30. [Google Scholar] [CrossRef]
- Borkosky, S.L.; Roukis, T.S. Incidence of Repeat Amputation after Partial First Ray Amputation Associated with Diabetes Mellitus and Peripheral Neuropathy: An 11-Year Review. J. Foot Ankle Surg. 2013, 52, 335–338. [Google Scholar] [CrossRef]
- Thorud, J.C.; Jupiter, D.C.; Lorenzana, J.; Nguyen, T.T.; Shibuya, N. Reoperation and Reamputation After Transmetatarsal Amputation: A Systematic Review and Meta-Analysis. J. Foot Ankle Surg. 2016, 55, 1007–1012. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.L.; Tardáguila-García, A.; García-Klepzig, J.L. Diagnostic and therapeutic update on diabetic foot osteomyelitis. Endocrinol. Diabetes Nutr. 2017, 64, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Baquie, M.; Mosser, S.; Herve, M.-P.; Cerdan-Bruyere, P.; Roux-Lombard, P.; Modoux Losberger, C.; Kocjancic Curty, L. Regenerative secretoma of adipose-derived stem cells from ischemic patients. J. Stem Cell Res. Ther. 2019, 9, 5. [Google Scholar]
- Uçkay, I.; Imhof, B.A.; Kressmann, B.; Lew, D.; Lipsky, B.A.; Sidibe, A. Characterization of Proangiogenic Monocytes from Blood in Patients with Chronic Ischemic Diabetic Foot Ulcers and Controls. Stem Cells Dev. 2020, 29, 911–918. [Google Scholar] [CrossRef] [PubMed]

| Infection Severity | Pathogens | Possible Antibiotics | Comments |
|---|---|---|---|
| Mild | Staphylococcus aureus (MSSA); Streptococcus spp. Methicillin-resistant S. aureus (MRSA) | Levofloxacin Amoxicillin-clavulanate Cephalexin Dicloxacillin Clindamycin Doxycycline Trimethoprim/ sulfamethoxazole | QD dosing; substandard for S. aureus Relatively broad spectrum & anti-anaerobic Requires QID dosing; inexpensive Narrow-spectrum; QID dosing; inexpensive Covers most (macrolide sensitive) MRSA & anaerobes MRSA, some gram-negatives; QD dosing MRSA, some gram-negatives; undefined against Streptococcus species |
| Moderate/Severe | MSSA; Streptococcus spp.; Enterobacteriaceae; obligate anaerobes MRSA Pseudomonas aeruginosa MRSA, Enterobacteriaceae, P. aeruginosa, anaerobes | Ertapenem * Ampicillin-sulbactam Imipenem-cilastatin (other carbapenems) Levofloxacin, or ciprofloxacin, with clindamycin Moxifloxacin Ceftriaxone Linezolid * Tigecycline Vancomycin Daptomycin Piperacillin-tazobactam * Vancomycin plus: - Piperacillin-tazobactam, or - Ceftazidime vs. cefepime, or - a carbapenem | QD dosing. Broad-spectrum anti-anaerobic; poor against Pseudomonas aeruginosa Relatively broad-spectrum but not for P. aeruginosa or other resistant gram-negatives Broad-spectrum; not active for MRSA; consider for proven/suspected ESBL producing pathogens Both oral and parenteral dosage forms suitable. Limited studies of clindamycin for severe S. aureus infections; possible anti-toxin effect QD doing. Broad-spectrum, including anaerobes QD dosing (IV or IM); 3rd gen. cephalosporin Oral and IV; adverse effects, drug interactions Broad-spectrum including MRSA; frequent gastrointestinal upset; less effective than others Narrow-spectrum; rising MICs in MRSA isolates QD-dosing; monitor CPK levels TID or QID dosing Very broad spectrum for empiric therapy in severe infections; narrow spectrum when culture & sensitivity results become available |
| Basic Approach to a Diabetic Person with Possible Foot Osteomyelitis. |
|---|
| Diagnosis - Clinical: wound size/depth; visible/palpable bone; soft tissue infection; PAD - Laboratory: WBC count; erythrocyte sedimentation rate; C-reative protein; procalcitonin - Imaging: Plain X-rays; advanced imaging if needed(MRI, radionuclide scans, PET/CT) - Cultures: Deep tissue specimens; bone specimen (surgical or transcutaneous) if possible |
| Treatment - Surgery - Urgent if needed for soft tissue debridement, or pus drainage - Elective in most cases if mainly for bone debridement, resection, or amputation - Preferred primary approach for patients with: exposed bone or joint; necrotic soft tissue; fluid collection or abscess; advanced bone destruction; need for other surgical repairs; lack of response to antibiotic treatment; high risk for antibiotic resistant pathogens or antibiotic-related toxicity - Antibiotics - Empirical: Broad-spectrum, or targeted if available culture results, while awaiting results of culture and antibiotic sensitivity tests - Definitive: Baseed on: culture and antibiotic sensitivity results; clinical response to empiric therapy; and, antibiotic stewardship principles - Preferred primary therapy for patients with: infection confined to the forefoot; adequate limb perfusion; no tissue necrosis; contraindications to, high risk from, or patient preference to avoid, surgery - Adjunctive: no treatments of proven benefit |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipsky, B.A.; Uçkay, İ. Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update. Medicina 2021, 57, 339. https://doi.org/10.3390/medicina57040339
Lipsky BA, Uçkay İ. Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update. Medicina. 2021; 57(4):339. https://doi.org/10.3390/medicina57040339
Chicago/Turabian StyleLipsky, Benjamin A., and İlker Uçkay. 2021. "Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update" Medicina 57, no. 4: 339. https://doi.org/10.3390/medicina57040339
APA StyleLipsky, B. A., & Uçkay, İ. (2021). Treating Diabetic Foot Osteomyelitis: A Practical State-of-the-Art Update. Medicina, 57(4), 339. https://doi.org/10.3390/medicina57040339







