Health Related Quality of Life T-14 Outcomes for Pediatric Bizact Tonsillectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design
2.3. Statistical Methods
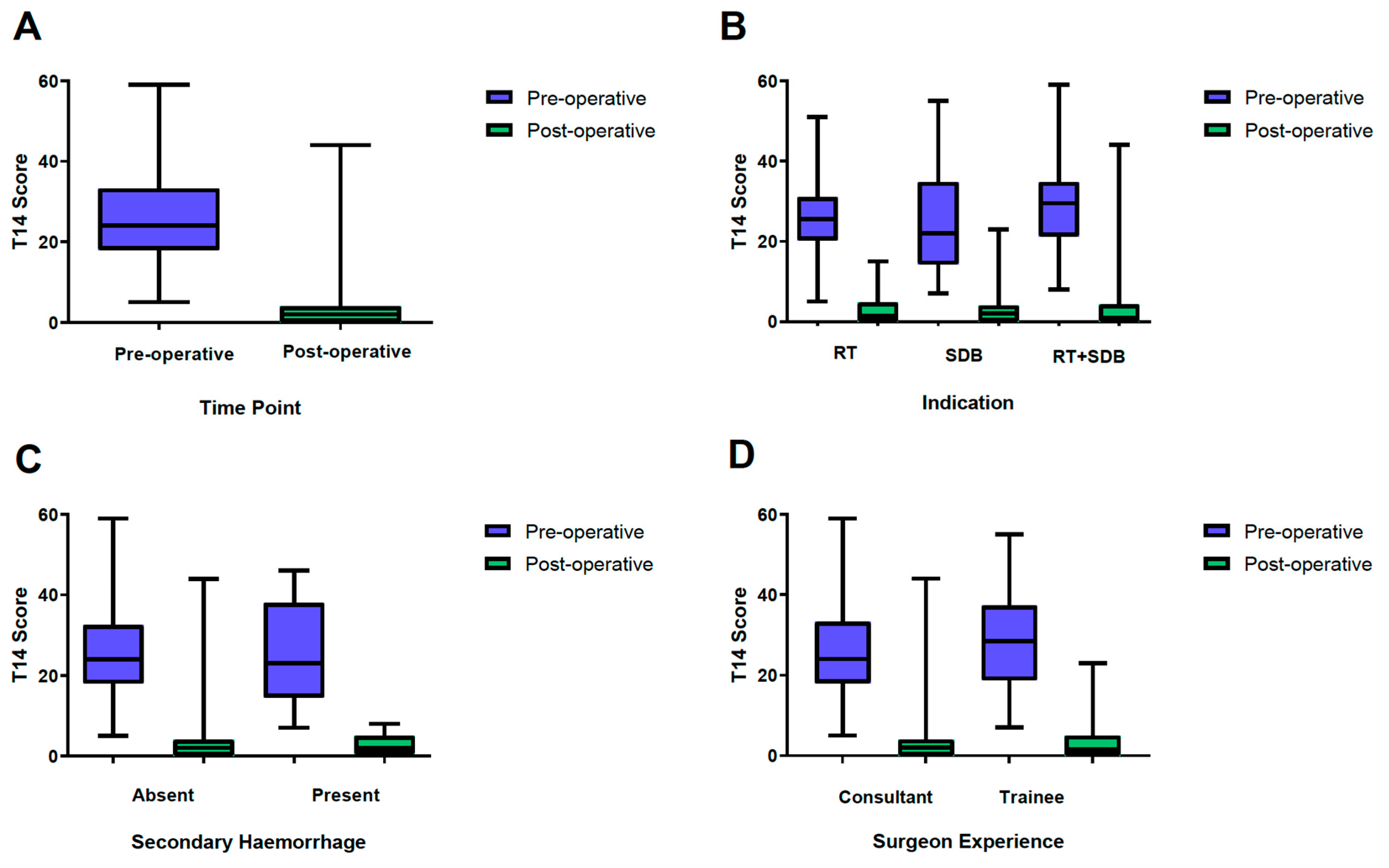
3. Results
3.1. Patient Demographics
3.2. T-14 Scoring
3.3. Surgeon Grade
3.4. Post-Tonsillectomy Hemorrhage
3.5. Post-Operative Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, G.; Stepan, L.; Du, C.; Padhye, V.; Bassiouni, A.; Dharmawardana, N.; Ooi, E.H.; Krishnan, S. Tonsillectomy using the BiZact: A pilot study in 186 children and adults. Clin. Otolaryngol. 2019, 44, 392–396. [Google Scholar] [CrossRef]
- Thong, G.; Davies, K.; Murphy, E.; Keogh, I. Significant improvements in quality of life following paediatric tonsillectomy: A prospective cohort study. Ir. J. Med. Sci. 2017, 186, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.A.; Gorynski, M.; Yip, C.; Harounian, J.; Huberman, H.; Weedon, J. Developmental delay in young children with sleep-disordered breathing before and after tonsil and adenoid surgery. Int. J. Pediatr. Otorhinolaryngol. 2016, 85, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.E.; Woods, C.M.; Du, C.; Milton, T.; Kao, S.S.-T.; Huynh, J.; Sigston, E.; Ooi, E.H. Outcomes using the T-14 symptom score for tonsillectomy in an Australian paediatric population. Aust. J. Otolaryngol. 2019, 2. [Google Scholar] [CrossRef]
- Hopkins, C.; Almeyda, R.; Alreefy, H.; Ismail-Koch, H.; Lim, J.; Possamai, V.; Powell, S.; Sharma, R.; Hore, I. Multicentre prospective clinical application of the T14 paediatric outcome tool. J. Laryngol. Otol. 2015, 129, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.S.; Peters, M.D.J.; Dharmawardana, N.; Stew, B.; Ooi, E.H. Scoping review of pediatric tonsillectomy quality of life assessment instruments. Laryngoscope 2017, 127, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, K.; Biggs, T.C.; Caldera, S. Application of the Paediatric Throat Disorders Outcome Test (T-14) for tonsillectomy and adenotonsillectomy. Ann. R Coll. Surg. Engl. 2013, 95, 410–414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paradise, J.L.; Bluestone, C.D.; Bachman, R.Z.; Colborn, D.K.; Bernard, B.S.; Taylor, F.H.; Rogers, K.D.; Schwarzbach, R.H.; Sylyvan, B.A.; Gilbert, M.D.; et al. Efficacy of tonsillectomy for recurrent throat infection in severely affected children. Results of parallel randomized and nonrandomized clinical trials. N. Engl. J. Med. 1984, 310, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Sarny, S.; Ossimitz, G.; Habermann, W.; Stammberger, H. Hemorrhage following tonsil surgery: A multicenter prospective study. Laryngoscope 2011, 121, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Soni-Jaiswal, A.; Anderco, I.; Kumar, B.N. Patient-reported outcomes in children suffering with mild to moderate tonsillitis versus those in children with severe tonsillitis. J. Laryngol. Otol. 2014, 128, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, K.M.; Pitts-Tucker, T.N.; Biggs, T.C.; Pringle, M.B. A five-year follow-up observational study of the T-14 paediatric throat disorders outcome measure in tonsillectomy and adenotonsillectomy. Ann. R Coll. Surg. Engl. 2019, 101, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Dawe, N.; Erskine, S.; Moor, J.W. The application and value of the ‘T-14 tool’ as a patient-reported outcome measure for paediatric tonsillectomy: A report on 45 cases. Clin. Otolaryngol. 2015, 40, 41–44. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Konieczny, K.M.; Biggs, T.C.; Pringle, M.B. A two-year follow-up observational study of the T-14 paediatric throat disorders outcome measure in tonsillectomy and adenotonsillectomy. Ann. R Coll. Surg. Engl. 2015, 97, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Soderman, A.C.; Odhagen, E.; Ericsson, E.; Hemlin, C.; Hultcrantz, E.; Sunnergren, O.; Stalfors, J. Post-tonsillectomy haemorrhage rates are related to technique for dissection and for haemostasis. An analysis of 15734 patients in the National Tonsil Surgery Register in Sweden. Clin. Otolaryngol. 2015, 40, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.; van der Meulen, J.; Cromwell, D.; Lewsey, J.; Copley, L.; Browne, J.; Yung, M.; Brown, P. Key messages from the National Prospective Tonsillectomy Audit. Laryngoscope 2007, 117, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.; Gillies, D. Post-tonsillectomy hemorrhage rates: Are they technique-dependent? Otolaryngol. Head Neck Surg. 2007, 136 (Suppl. 4), S27–S31. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.A.; Frauenfelder, C.; Woods, C.; Wee, C.; Carney, A.S. Bleeding following coblation tonsillectomy: A 10-year, single-surgeon audit and modified grading system. J. Laryngol. Otol. 2015, 129 (Suppl. 1), S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Ozkiris, M. Comparison of three techniques in pediatric tonsillectomy. Eur. Arch. Otorhinolaryngol. 2012, 269, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Hadjisymeou, S.; Modayil, P.C.; Dean, H.; Jonas, N.E.; Tweedie, D.J. Our experience. Coblation(R) intracapsular tonsillectomy (tonsillotomy) in children: A prospective study of 100 consecutive cases. Clin. Otolaryngol. 2014, 39, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, E.; Ericsson, E.; Hemlin, C.; Hessen-Soderman, A.C.; Roos, K.; Sunnergren, O.; Stalfors, J. Paradigm shift in Sweden from tonsillectomy to tonsillotomy for children with upper airway obstructive symptoms due to tonsillar hypertrophy. Eur. Arch. Otorhinolaryngol. 2013, 270, 2531–2536. [Google Scholar] [CrossRef] [PubMed]
| Demographics | N = 146 |
|---|---|
| Male: Female (number) | 76:70 |
| Age range (years) | 1–16 |
| Median age (years) | 5.5 |
| Indication | |
| Sleep Disordered breathing (SDB)(number) | 84 |
| Recurrent Infection (RT) (number) | 28 |
| SDB and Infection (SDB and RT) (number) | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepan, L.; Huang, L.; Huynh, J.; Xie, P.; Woods, C.M.; Ooi, E.H. Health Related Quality of Life T-14 Outcomes for Pediatric Bizact Tonsillectomy. Medicina 2021, 57, 480. https://doi.org/10.3390/medicina57050480
Stepan L, Huang L, Huynh J, Xie P, Woods CM, Ooi EH. Health Related Quality of Life T-14 Outcomes for Pediatric Bizact Tonsillectomy. Medicina. 2021; 57(5):480. https://doi.org/10.3390/medicina57050480
Chicago/Turabian StyleStepan, Lia, Lucy Huang, Julie Huynh, Phillip Xie, Charmaine M. Woods, and Eng H. Ooi. 2021. "Health Related Quality of Life T-14 Outcomes for Pediatric Bizact Tonsillectomy" Medicina 57, no. 5: 480. https://doi.org/10.3390/medicina57050480
APA StyleStepan, L., Huang, L., Huynh, J., Xie, P., Woods, C. M., & Ooi, E. H. (2021). Health Related Quality of Life T-14 Outcomes for Pediatric Bizact Tonsillectomy. Medicina, 57(5), 480. https://doi.org/10.3390/medicina57050480







