Acute Pericarditis after Percutaneous Coronary Intervention: A Case Report
Abstract
:1. Introduction
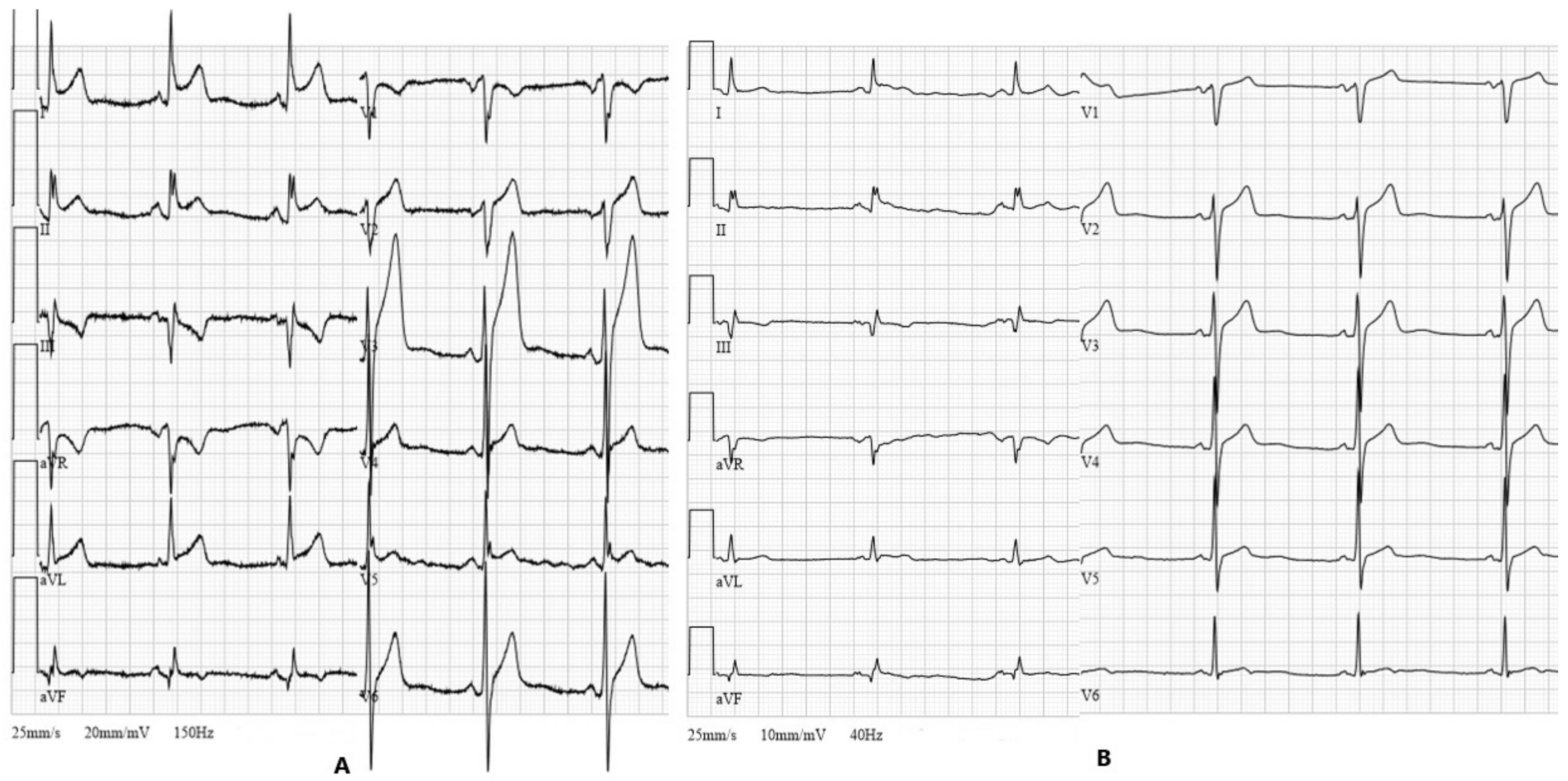
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2921–2964. [Google Scholar] [CrossRef]
- Kytö, V.; Sipilä, J.; Rautava, P. Clinical Profile and Influences on Outcomes in Patients Hospitalized for Acute Pericarditis. Circulation 2014, 130, 1601–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imazio, M.; Spodick, D.H.; Brucato, A.; Trinchero, R.; Adler, Y. Controversial Issues in the Management of Pericardial Diseases. Circulation 2010, 121, 916–928. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Hoit, B.D. Post-cardiac injury syndromes. An emerging cause of pericardial diseases. Int. J. Cardiol. 2013, 168, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Sasse, T.; Eriksson, U. Post-cardiac injury syndrome: Aetiology, diagnosis, and treatment. ESC E-J. Cardiol. Pract. 2017, 15, 21–31. [Google Scholar]
- Holmes, D.R.; Nishimura, R.; Fountain, R.; Turi, Z.G. Iatrogenic pericardial effusion and tamponade in the percutaneous intracardiac intervention era. JACC Cardiovasc. Interv. 2009, 2, 705–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troughton, R.W.; Asher, C.R.; Klein, A.L. Pericarditis. Lancet 2004, 363, 717–727. [Google Scholar] [CrossRef]
- Elbaz-Greener, G.; Wijeysundera, H.C. A presentation of postcardiac injury syndrome after successful chronic total occlusion percutaneous coronary intervention using dissection re-entry techniques. Clin. Case Rep. 2017, 5, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, K.; Matsumoto, M.; Sugita, T.; Nishizawa, J.; Yoshioka, T.; Tokuda, Y.; Ueda, Y. Clinical characteristics of patients with constrictive pericarditis after coronary bypass surgery. Jpn. Circ. J. 2001, 65, 480–482. [Google Scholar] [CrossRef] [Green Version]
- Escaned, J.; Ahmad, R.A.; Shiu, M.F. Pleural effusion following coronary perforation during balloon angioplasty: An unusual presentation of the postpericardiotomy syndrome. Eur. Heart J. 1992, 13, 716–717. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-J.; Kuo, L.-T.; Cherng, W.-J. Acute pericarditis following percutaneous transluminal coronary intervention—A case report. Angiology 2003, 54, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-P.; Yu, W.-C.; Lu, T.-M. Acute pericarditis after percutaneous coronary intervention mimicking inferolateral ST-elevation myocardial infarction. J. Invasive Cardiol. 2013, 25, E27–E29. [Google Scholar] [PubMed]
- Imazio, M.; Brucato, A.; Cemin, R.; Ferrua, S.; Maggiolini, S.; Beqaraj, F.; Demarie, D.; Forno, D.; Ferro, S.; Maestroni, S.; et al. A randomized trial of colchicine for acute pericarditis. N. Engl. J. Med. 2013, 369, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.F. Acute pericarditis: Update on diagnosis and management. Clin. Med. 2020, 20, 48–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodevič, G.; Budrys, P.; Davidavičius, G. Acute Pericarditis after Percutaneous Coronary Intervention: A Case Report. Medicina 2021, 57, 490. https://doi.org/10.3390/medicina57050490
Rodevič G, Budrys P, Davidavičius G. Acute Pericarditis after Percutaneous Coronary Intervention: A Case Report. Medicina. 2021; 57(5):490. https://doi.org/10.3390/medicina57050490
Chicago/Turabian StyleRodevič, Greta, Povilas Budrys, and Giedrius Davidavičius. 2021. "Acute Pericarditis after Percutaneous Coronary Intervention: A Case Report" Medicina 57, no. 5: 490. https://doi.org/10.3390/medicina57050490
APA StyleRodevič, G., Budrys, P., & Davidavičius, G. (2021). Acute Pericarditis after Percutaneous Coronary Intervention: A Case Report. Medicina, 57(5), 490. https://doi.org/10.3390/medicina57050490




