Abstract
Background and Objective: Patients with advanced non-small-cell lung cancer (NSCLC) harboring sensitizing epidermal growth factor receptor (EGFR) mutations show a good response to EGFR-tyrosine kinase inhibitors (EGFR-TKIs). The subsequent treatments influence the evaluability of the efficacy of front-line therapy on overall survival (OS). Consequently, we evaluated the associations of relapse-free survival (RFS) and post-progression survival (PPS) with OS in patients who exhibited postoperative relapse of EGFR-mutated NSCLC. Materials and Methods: We analyzed the data of 35 patients with EGFR-mutated NSCLC who underwent complete resection between January 2007 and June 2019. The correlations of RFS and PPS with OS were evaluated at the individual patient level. Results: Linear regression and Spearman’s rank correlation analyses demonstrated that the PPS highly correlated with OS (r = 0.91, p < 0.05, R2 = 0.85), whereas the RFS weakly associated with OS (r = 0.36, p < 0.05, R2 = 0.25). Age and performance status at relapse were significantly associated with PPS. Conclusion: Overall, PPS was more strongly and significantly associated with OS than RFS. These results suggest that the OS of our cohort may be affected by treatments, besides postoperative relapse. However, larger-scale prospective studies are needed to confirm these results.
1. Introduction
Lung cancer is a major reason for cancer-related mortality globally, and non-small-cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers [1]. For early stage NSCLC, surgical resection is considered the most effective strategy, with the highest potential for improving survival and cure. However, despite complete resection, recurrence and death occur in approximately half of the cases with stage I–IIIA NSCLC [2,3]. It is highly unlikely that postoperative relapse of NSCLC is curable, and the median survival beyond relapse is 8.1–17.7 months [3,4]. An optimal therapeutic strategy for postoperative relapse of NSCLC is expected to alleviate clinical symptoms, maintain quality of life, and slow down disease progression.
With the increasing number of treatment options for NSCLC, the efficacy of front-line therapy on overall survival (OS) might be affected by subsequent treatments [5]. A phase III trial demonstrated that prolonged progression-free survival (PFS) does not always result in prolonged OS of patients with NSCLC [6]. Thus, PFS after first-line therapy is not an ideal alternative endpoint for OS. Instead, post-progression survival (PPS), which is calculated as the difference between OS and PFS, is reportedly highly correlated with OS following first-line therapy with molecular targeted agents, such as EGFR-tyrosine kinase inhibitors (TKIs) [7,8,9]. Previously, we reported that PPS has a stronger significance on OS than PFS in patients with NSCLC harboring sensitizing epidermal growth factor receptor (EGFR) mutations treated with first-line EGFR-TKIs. This means that treatment beyond disease progression after front-line treatment may have a significant influence on the OS of patients with NSCLC [10].
The clinical characteristics and prognoses of patients with EGFR-mutated NSCLC versus those without EGFR mutations have been comprehensively investigated [11,12]. Numerous clinical trials have shown the effectiveness of EGFR-TKIs, such as gefitinib, erlotinib, afatinib, and osimertinib, as a first-line therapy for patients harboring sensitizing EGFR mutations [13,14,15,16,17,18,19]. In addition to first-line EGFR-TKIs, other therapeutic choices include platinum-based combination regimens and non-platinum regimens. Approximately 60% of patients who progress after the first-line therapy with a first- or second-generation EGFR-TKI harbor a T790M mutation in EGFR [20,21,22,23]. Osimertinib is one of the standard second-line therapy choices for patients with progressive T790M-positive NSCLC following relapse beyond first-line therapy with a first- or second-generation EGFR-TKI [24]. Patients with metastatic T790M-negative NSCLC who progress beyond first-line therapy with a first- or second-generation EGFR-TKI are treated with cytotoxic drugs. However, despite recent large-cohort studies, the clinical and prognostic implications of EGFR mutation status in surgically resected lung cancer remain controversial [12,25,26].
It would be interesting to examine the contribution of postoperative relapse treatment to OS at the individual-level. An evaluation of individual patient-level data demonstrated that PPS, and not PFS, is strongly correlated with OS beyond front-line therapy in patients with metastatic NSCLC and small-cell lung cancer (SCLC) [27]. Consequently, continuing therapy beyond postoperative relapse may significantly influence OS. However, the correlation between PPS and OS in postoperative relapse of EGFR-mutated NSCLC is currently unclear. Therefore, evaluating individual patient-level data to determine whether relapse-free survival (RFS) and PPS are considerably correlated with OS beyond postoperative relapse is of clinical relevance.
In the current investigation, we evaluated the relationships of RFS and PPS with OS in postoperative relapse patients with NSCLC haboring sensitizing EGFR mutations. In addition, we assessed the prognostic significance of a patient’s characteristics for PPS.
2. Patients and Methods
2.1. Patients
The current study involved patients with postoperative relapse of EGFR-mutated NSCLC who underwent a complete resection at Gunma Prefectural Cancer Center between January 2007 and June 2019. The histopathological diagnosis was determined according to the World Health Organization’s classification. The NSCLC stage was determined based on the American Joint Committee on Cancer’s tumor-node-metastasis (TNM) staging system [28]. The inclusion criteria were histologically proven NSCLC, postoperative relapse, and a carcinoma harboring sensitizing EGFR mutations (exon 18 G719X, exon 19 deletion, exon 21 L858R, or exon 21 L861Q). In addition, only lobectomies were included, not wedge resection or segmentectomy. Lymph node dissection was also included in this study. On the other hand, the exclusion criteria were operations in other hospitals, incomplete resection, and incomplete data. At postoperative relapse, before treatment, each patient underwent a physical examination, a chest radiograph, a computed tomography of the chest and abdomen, a 18F-fluorodeoxyglucose positron emission tomography or bone scintigraphy, and a brain computed tomography or magnetic resonance imaging to determine the disease stage (TNM classification). The medical records of the identified patients were collected and checked. Furthermore, records on patient characteristics, chemotherapeutic regimens, radiotherapy, and subsequent-line treatments (if administered) were collected. First- and higher-line treatments were chosen by the principal medical oncologist and continued until disease progression, unacceptable toxicities, or treatment refusal. Beyond operative recurrence, the patients were permitted to choose any treatment modality following the first-line therapy.
Sensitizing EGFR mutations in exons 18–21 were evaluated, as previously demonstrated [29,30], by polymerase chain reaction (PCR) amplification with intron–exon boundary primers. Four sensitizing EGFR mutations were identified, of which exon 19 deletion and exon 21 L858R were the major sensitizing mutations, whereas exon 18 G719X and exon 21 L861Q were the minor sensitizing mutations.
This study was approved by the ethics committee of Gunma Prefectural Cancer Center. Owing to the retrospective nature of the study, the requirement for informed consent was waived by the ethics committee. However, the opportunity to refuse participation through an opt-out method was guaranteed.
2.2. Treatment Response Assessment
Tumor response was evaluated as the best overall response. Radiological tumor responses were evaluated according to RECIST version 1.1 [31]: disappearance of all target lesions (complete response, CR); a ≥ 30% decrease in the sum of target lesion diameters relative to the baseline level (partial response, PR); a ≥ 20% increase in the sum of target lesion diameters relative to the smallest value during the study (progressive disease, PD); and insufficient shrinkage to qualify as PR and insufficient growth to qualify as PD (stable disease, SD).
2.3. Statistical Analysis
We defined RFS as the time from operation to the first instance of relapse or death from any reason. Overall survival was defined as the time from operation until death or censoring at the last consultation. We defined PPS as the time from tumor relapse after operation until death or censoring at the last consultation. Survival curves were generated using the Kaplan–Meier method. Linear regression analyses and Spearman’s rank correlation coefficient were adopted to assess whether RFS and/or PPS were associated with OS. A Cox proportional hazards model with stepwise regression was adopted to identify factors that predicted PPS and to estimate hazard ratios and 95% confidence intervals. Some variables were converted to an appropriate scale unit because the hazard ratio was calculated based on a 1-unit difference. Differences were regarded as statistically significant at a two-tailed p-value of ≤0.05. All analyses were conducted using JMP software for Windows, version 11.0 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Patient Baseline Characteristics and Therapeutic Efficacy
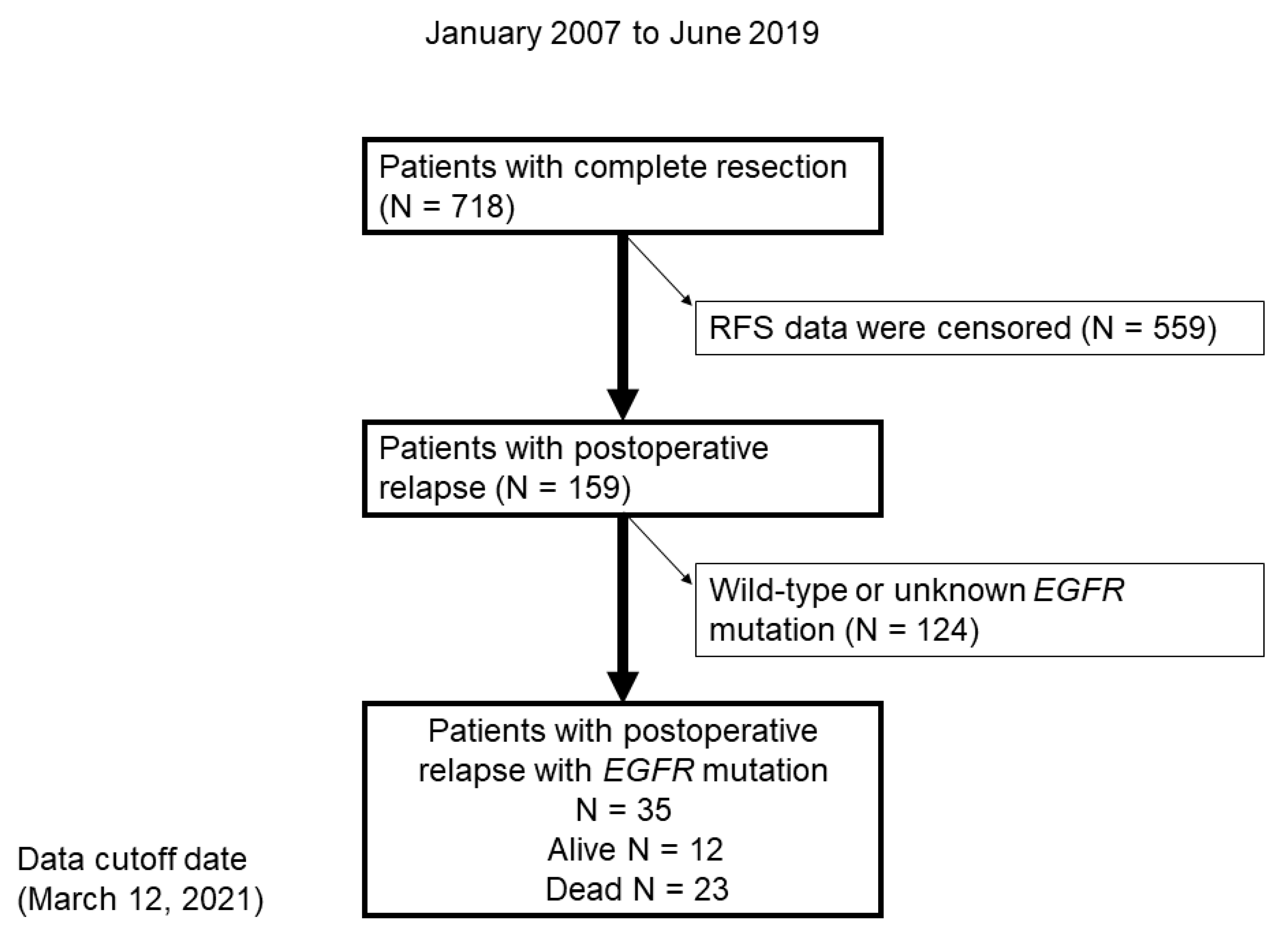
In total, 718 patients underwent a complete resection, 159 of whom had postoperative relapse. Subsequently, 124 patients with wild-type EGFR or unknown EGFR mutation status were excluded (Figure 1). Finally, 35 patients with EGFR-positive NSCLC were included in the study.
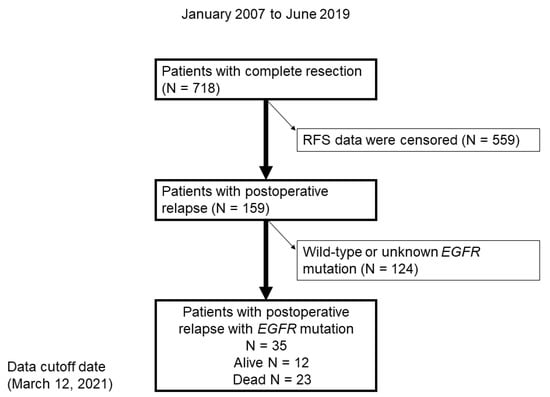
Figure 1.
Flow chart demonstrating the identification of patients with postoperative relapse of NSCLC with EGFR mutation between January 2007 and June 2019. RFS, relapse-free survival.
Of the 35 patients whose data were analyzed in this study, 23 died because of underlying diseases, and 12 are alive. The median follow-up period was 51.6 (range, 11.6–146.5) months. The median patient age was 69 years (range, 44–83 years). The baseline characteristics of the patients are listed in Table 1.

Table 1.
Baseline characteristics of the patients.
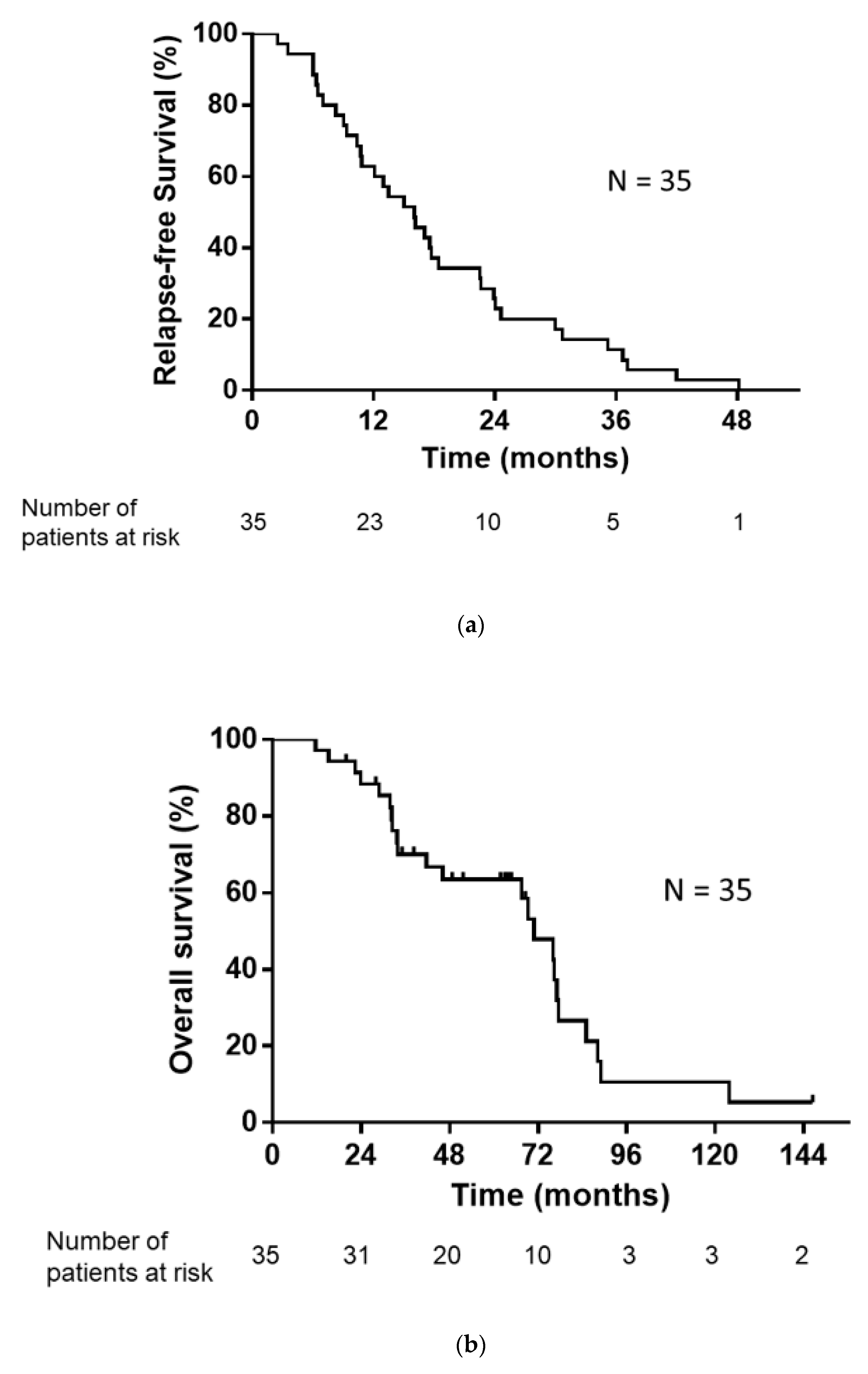
The median number of regimens after postoperative relapse for the 35 patients was 1 (range, 0–7). The treatments administered following postoperative relapse are listed in Table 2. Of the 35 patients with postoperative relapse, 34 patients (excluding one patient who received only supportive care) received some form of drug therapy or radiotherapy. As an initial treatment following postoperative relapse, 26 patients received EGFR-TKI and three patients received cytotoxic drugs. Five patients underwent definitive thoracic irradiation, including one patient who received concurrent chemoradiotherapy. The median RFS, PPS, and OS were 16.0, 52.2, and 70.9 months, respectively (Figure 2a,b).

Table 2.
Treatments after postoperative relapse.
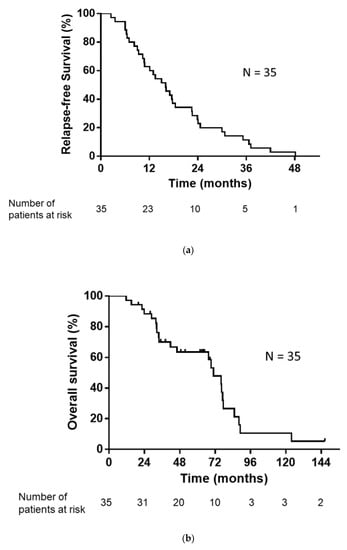
Figure 2.
(a) Kaplan–Meier plot showing relapse-free survival (RFS). Median RFS: 16.0 months. (b) Kaplan–Meier plot showing overall survival (OS). Median OS: 70.9 months.
3.2. Correlations of RFS and PPS with OS
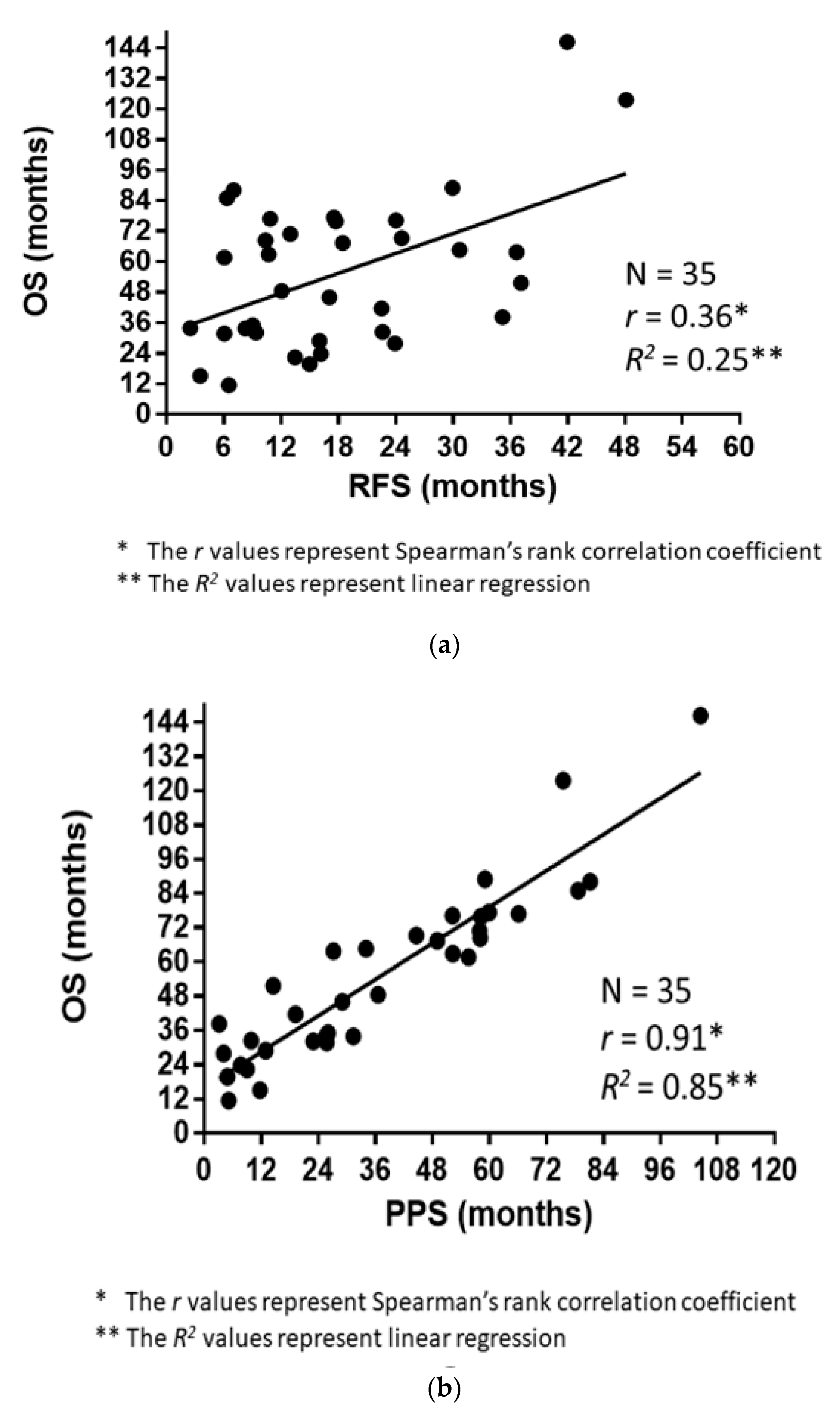
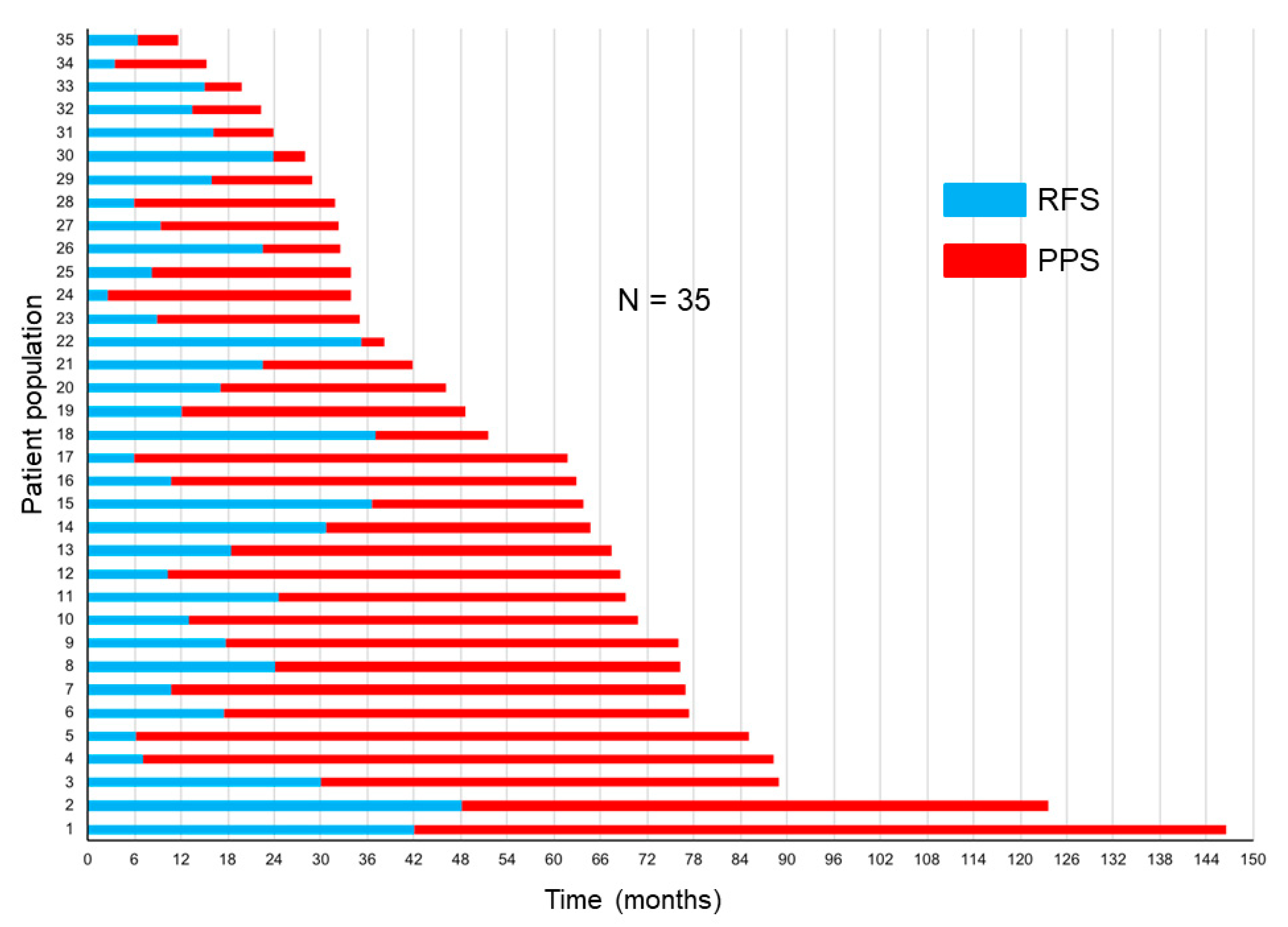
The correlations of RFS and PPS with OS are demonstrated in Figure 3a,b. The Spearman’s rank correlation coefficients and linear regression revealed that the PPS strongly correlated with OS (r = 0.91, p < 0.0001, R2 = 0.85), whereas the RFS did not (r = 0.36, p = 0.03, R2 = 0.25). Figure 4 demonstrates the RFS and PPS of the entire study population.
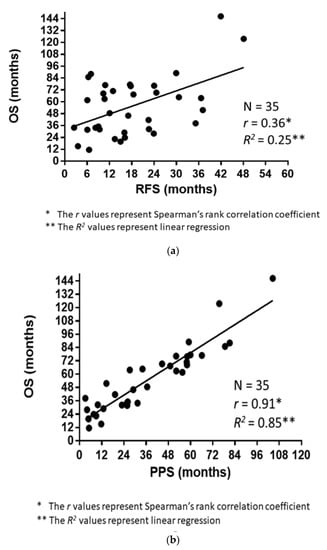
Figure 3.
(a) Association between the overall survival (OS) and relapse-free survival (RFS). (b) Association between the overall survival (OS) and post-progression survival (PPS).
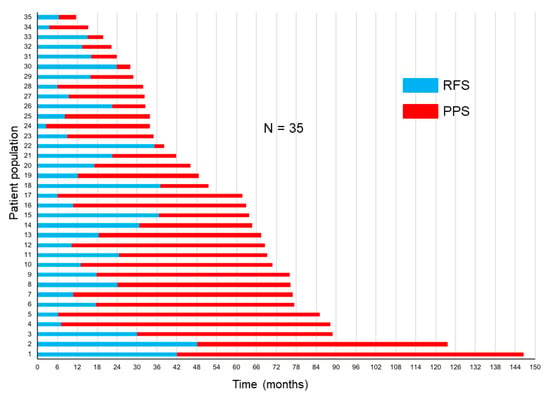
Figure 4.
Relapse-free survival (RFS) and post-progression survival (PPS) in the entire study population.
3.3. Clinical Factors Affecting PPS
The univariate Cox regression analysis demonstrated that age at relapse, performance status (PS) at relapse, treatment with or without adjuvant chemotherapy, and presence of bone metastases at relapse were significantly associated with PPS (p < 0.05) (Table 3). In addition, the multivariate Cox regression analysis demonstrated that age at relapse and PS at relapse significantly influenced the PPS (p < 0.05, Table 3).

Table 3.
Univariate and multivariate analyses of patient backgrounds for post-progression survival.
4. Discussion
We evaluated the correlations of OS with RFS and PPS at the individual patient level in patients who exhibited postoperative relapse of NSCLC harboring sensitizing EGFR mutations. The PPS was more significantly correlated with OS than RFS. Furthermore, age at relapse and PS at relapse were significantly associated with PPS. Most patients with postoperative relapse received regular follow-ups beyond surgical resection; therefore, the tumor burden might be lower than that in patients with metastatic NSCLC at initial diagnosis. These discrepancies in the burden and heterogeneity may favor PFS and OS in patients with postoperative relapse NSCLC [32,33,34].
Various trial endpoints have been examined in meta-analyses [35,36], and biostatisticians have suggested numerous assessment criteria to confirm alternative endpoints [37,38]. One study revealed that PPS, which was defined as survival post-progression, is significant for evaluating the adequacy of OS as a study endpoint [9]. PFS is generally defined as the survival time without “progression or death for any reason” after surgery. Relapse-free survival is generally defined as the survival time without “relapse or death for any reason” in the disease-free (cancer-free) state after a surgery. Other studies in patients with NSCLC have also demonstrated that PPS is highly correlated with OS beyond first-, second-, or third-line therapy [7,8,39]. Moreover, our previous studies revealed that patient-level data on PPS are relevant for evaluating early (first- and second-line) therapies in patients with advanced or metastatic NSCLC, as well as first-line treatment in patients with extensive-disease SCLC [10,40,41,42,43]. Therefore, in this study, we analyzed RFS and PPS in patients with postoperative relapse of EGFR-mutated NSCLC. Our findings demonstrated that RFS did not consistently correlate with OS, suggesting it may not be a valuable marker for prolonged OS. In addition, RFS was considerably shorter than PPS in the current cohort. PPS was highly associated with OS, suggesting that future clinical studies should consider the factors that may influence PPS.
A previous study of NSCLC has shown that prolonged PPS with first-line monotherapy and molecularly targeted drugs is strongly correlated with favorable PS [7]; though, the clinical factors influencing PPS at the individual patient-level in the postoperative relapse of NSCLC with sensitizing EGFR mutations remains unclear. In the current study, the multivariate analysis revealed that the following two factors were closely correlated with PPS: age at relapse and PS at relapse. This observation indicates that age at relapse and PS at relapse in patients with postoperative relapse of EGFR-mutated NSCLC may be important for prolonging PPS. The high number of anticancer agents administered beyond postoperative relapse can be attributed to the wide availability of first- and subsequent-line treatment options for NSCLC, including EGFR-TKIs (gefitinib, erlotinib, afatinib, and osimertinib), platinum-based combination regimen, pemetrexed, docetaxel, S1, and immune checkpoint inhibitors (ICIs) (Table 2). Osimertinib (a third-generation EGFR-TKI) demonstrated better drug-toxicity profiles than first- and second-generation EGFR-TKIs in trials, and their efficacies in patients with advanced or metastatic NSCLC with secondary T790M mutation and EGFR-TKI resistance are encouraging [20]. Although most cases in our cohort died before the evaluation of T790M mutation, if several patients with a secondary T790M EGFR-mutation are treated with osimertinib, the influence on PPS could be stronger than currently anticipated. Osimertinib use correlated with better PFS than current standard first-line therapies in patients harboring sensitizing EGFR mutations [19]. Therefore, osimertinib might be a more reliable standard front-line therapy for patients harboring sensitizing EGFR mutations. As first- and subsequent-line treatments are undergoing changes, the PPS beyond postoperative relapse in these patients might also show a change. Post-progression survival has a greater influence on the OS of patients with NSCLC harboring secondary T790M mutation when osimertinib is administered as a second-line therapy, in addition to first-line treatment with first- or second-generation EGFR-TKIs. However, PPS may be of value when using osimertinib as a first-line treatment after postoperative relapse. Current analyses imply that OS is more highly correlated with PPS than RFS in patients with EGFR-mutated NSCLC who underwent complete resection. Therefore, subsequent therapies might prolong the OS of these patients. The univariate analysis showed that the presence of a T790M mutation and the administration of osimertinib in the first-line treatment and ICIs were not statistically significant for PPS in the current analysis; however, this could be attributed to the small cohort scale.
The current analysis has some limitations. First, the cohort size was small. This limited our capacity to assess the relationships among PPS, the presence of T790M mutation, and the administration of osimertinib in first-line treatment and immune checkpoint inhibitors. Only a small number of postoperative relapse patients with EGFR mutations were available at our institution. Moreover, we tried to assess patients with the same backgrounds. Although a relatively large number of these patients were treated at our institution, our clinical practices and strategies are largely uniform. Adjusting for various sources of bias in the current analysis ensured clinically relevant results. Future studies with a higher number of patients are needed. Second, the treatment methods after postoperative relapse were not uniform, varying from drug therapy to radiotherapy alone. Nevertheless, this study, which is based on the actual clinical course of treatment, is of clinical relevance. Third, the date of response was determined by different treating physicians, and this may have resulted in a variability in the RFS; however, this is a limitation inherent to retrospective analyses. Fourth, we also managed to obtain censored survival data, although this does not affect the conclusions. Even when patients did not reach the death event, the RFS did not change. Besides, PPS and OS were prolonged, and PPS was closely correlated with OS.
In conclusion, PPS is more highly associated with OS than RFS in patients with postoperative relapse of EGFR-mutated NSCLC. Age at relapse and PS at relapse were also significantly associated with PPS. These outcomes imply that the course of treatment after postoperative relapse influences the OS of patients with EGFR-mutated NSCLC, though larger-scale prospective studies are needed to confirm these results in other clinical situations and patient cohorts.
Author Contributions
H.I. and K.K. drafted the manuscript. H.I., R.O., K.K., M.G., D.K., and K.M. conceived and planned the study. H.I. and R.O. performed the data entry. H.I. and K.K. conducted the statistical analyses. M.G. and K.M. edited and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the ethics committee of the Institutional Review Board of the Gunma Prefectural Cancer Center. All procedures complied with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any animal studies performed by any of the authors.
Informed Consent Statement
Due to the retrospective nature of the study, the requirement for informed consent was waived by the ethics committee. However, the opportunity to refuse participation through an opt-out method was guaranteed.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available.
Acknowledgments
The authors appreciate Hiromi Sakamoto, Tomoko Nakajima, Emiko Kimura, Eri Kogure, and Sayaka Kawashima for their assistance in preparing this research.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Arriagada, R.; Bergman, B.; Dunant, A.; Le Chevalier, T.; Pignon, J.-P.; Vansteenkiste, J. International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-Based Adjuvant Chemotherapy in Patients with Completely Resected Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2004, 350, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, H.; Nichols, F.C.; Yang, P.; Allen, M.S.; Cassivi, S.D.; Deschamps, C.; Williams, B.A.; Pairolero, P.C. Survival After Recurrent Nonsmall-Cell Lung Cancer After Complete Pulmonary Resection. Ann. Thorac. Surg. 2007, 83, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Saisho, S.; Yasuda, K.; Maeda, A.; Yukawa, T.; Okita, R.; Hirami, Y.; Shimizu, K.; Nakata, M. Post-recurrence survival of patients with non-small-cell lung cancer after curative resection with or without induction/adjuvant chemotherapy. Interact. Cardiovasc. Thorac. Surg. 2012, 16, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.C.; Massard, C.; Le Chevalier, T. Should progression-free survival be the primary measure of efficacy for advanced NSCLC therapy? Ann. Oncol. 2010, 21, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Von Pawel, J.; Zatloukal, P.; Ramlau, R.; Gorbounova, V.; Hirsh, V.; Leighl, N.; Mezger, J.; Archer, V.; Moore, N.; et al. Phase III Trial of Cisplatin Plus Gemcitabine with Either Placebo or Bevacizumab as First-Line Therapy for Nonsquamous Non–Small-Cell Lung Cancer: AVAiL. J. Clin. Oncol. 2009, 27, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Kiura, K.; Fujiwara, Y.; Takigawa, N.; Hisamoto, A.; Ichihara, E.; Tabata, M.; Tanimoto, M. Role of survival post-progression in phase III trials of systemic chemotherapy in advanced non-small-cell lung cancer: A systematic review. PLoS ONE 2011, 6, e26646. [Google Scholar] [CrossRef]
- Hayashi, H.; Okamoto, I.; Morita, S.; Taguri, M.; Nakagawa, K. Postprogression survival for first-line chemotherapy of patients with advanced non-small-cell lung cancer. Ann. Oncol. 2012, 23, 1537–1541. [Google Scholar] [CrossRef]
- Broglio, K.R.; Berry, D.A. Detecting an Overall Survival Benefit that Is Derived from Progression-Free Survival. J. Natl. Cancer Inst. 2009, 101, 1642–1649. [Google Scholar] [CrossRef]
- Imai, H.; Kaira, K.; Mori, K.; Kotake, M.; Mitani, M.; Kawashima, N.; Hisada, T.; Minato, K. Post-progression survival is highly linked to overall survival in patients with non-small-cell lung cancer harboring sensitive EGFR mutations treated with first-line epidermal growth factor receptor-tyrosine kinase inhibitors. Thorac. Cancer 2019, 10, 2200–2208. [Google Scholar] [CrossRef]
- Kosaka, T.; Yatabe, Y.; Onozato, R.; Kuwano, H.; Mitsudomi, T. Prognostic implication of EGFR, KRAS, and TP53 gene mu-tations in a large cohort of Japanese patients with surgically treated lung adenocarcinoma. J. Thorac. Oncol. 2009, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Takamochi, K.; Oh, S.; Matsunaga, T.; Suzuki, K. Prognostic impacts of EGFR mutation status and subtype in patients with surgically resected lung adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2017, 154, 1768–1774.e1. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef]
- Maemondo, M.; Inoue, A.; Kobayashi, K.; Sugawara, S.; Oizumi, S.; Isobe, H.; Gemma, A.; Harada, M.; Yoshizawa, H.; Kinoshita, I.; et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010, 362, 2380–2388. [Google Scholar] [CrossRef]
- Mitsudomi, T.; Morita, S.; Yatabe, Y.; Negoro, S.; Okamoto, I.; Tsurutani, J.; Seto, T.; Satouchi, M.; Tada, H.; Hirashima, T.; et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010, 11, 121–128. [Google Scholar] [CrossRef]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann. Oncol. 2015, 26, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two random-ised, phase 3 trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Kobayashi, S.; Boggon, T.J.; Dayaram, T.; Janne, P.A.; Kocher, O.; Meyerson, M.; Johnson, B.E.; Eck, M.J.; Tenen, D.G.; Halmos, B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2005, 352, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Arcila, M.E.; Sima, C.S.; Riely, G.J.; Chmielecki, J.; Kris, M.G.; Pao, W.; Ladanyi, M.; Miller, V.A. Acquired re-sistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: Distinct natural history of patients with tumors har-boring the T790M mutation. Clin. Cancer Res. 2011, 17, 1616–1622. [Google Scholar] [CrossRef]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef]
- Yu, H.A.; Arcila, M.E.; Rekhtman, N.; Sima, C.S.; Zakowski, M.F.; Pao, W.; Kris, M.G.; Miller, V.A.; Ladanyi, M.; Riely, G.J. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. 2013, 19, 2240–2247. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.L.; Ahn, M.J.; Garassino, M.C.; Kim, H.R.; Ramalingam, S.S.; Shepherd, F.A.; He, Y.; Akamatsu, H.; Theelen, W.S.; et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N. Engl. J. Med. 2017, 376, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Miyata, Y.; Tsutani, Y.; Ito, H.; Nakayama, H.; Imai, K.; Ikeda, N.; Okada, M. Positive EGFR mutation status is a risk of recurrence in pN0–1 lung adenocarcinoma when combined with pathological stage and histological subtype: A retrospective multi-center analysis. Lung Cancer 2020, 141, 107–113. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Xin, P.; Zhang, M.; Jiang, S.; Zhang, J.; Zhong, S.; Liu, Y.; Guo, M.; Chen, X.; Xia, X.; et al. The impact of epidermal growth factor receptor mutations on the prognosis of resected non-small cell lung cancer: A meta-analysis of literatures. Transl. Lung Cancer Res. 2019, 8, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kaira, K.; Minato, K. Clinical significance of post-progression survival in lung cancer. Thorac. Cancer 2017, 8, 379–386. [Google Scholar] [CrossRef]
- Goldstraw, P.; Crowley, J.; Chansky, K.; Giroux, D.J.; Groome, P.A.; Rami-Porta, R.; Postmus, P.E.; Rusch, V.; Sobin, L. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the TNM Stage Groupings in the Forthcoming (Seventh) Edition of the TNM Classification of Malignant Tumours. J. Thorac. Oncol. 2007, 2, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Nagai, Y.; Miyazawa, H.; Huqun; Tanaka, T.; Udagawa, K.; Kato, M.; Fukuyama, S.; Yokote, A.; Kobayashi, K.; Kanazawa, M.; et al. Genetic Heterogeneity of the Epidermal Growth Factor Receptor in Non–Small Cell Lung Cancer Cell Lines Revealed by a Rapid and Sensitive Detection System, the Peptide Nucleic Acid-Locked Nucleic Acid PCR Clamp. Cancer Res. 2005, 65, 7276–7282. [Google Scholar] [CrossRef] [PubMed]
- Yatabe, Y.; Hida, T.; Horio, Y.; Kosaka, T.; Takahashi, T.; Mitsudomi, T. A Rapid, Sensitive Assay to Detect EGFR Mutation in Small Biopsy Specimens from Lung Cancer. J. Mol. Diagn. 2006, 8, 335–341. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tu-mours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Taniguchi, K.; Okami, J.; Kodama, K.; Higashiyama, M.; Kato, K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci. 2008, 99, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Reis-Filho, J.S. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012, 13, e178–e185. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, T.M.; Keam, B.; Jeon, Y.K.; Lee, S.-H.; Kim, D.-W.; Chung, D.H.; Kim, Y.T.; Kim, Y.W.; Heo, D.S. Tumor Burden is Predictive of Survival in Patients with Non–Small-Cell Lung Cancer and With Activating Epidermal Growth Factor Receptor Mutations Who Receive Gefitinib. Clin. Lung Cancer 2013, 14, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.R.; Ringland, C.; Stokes, B.J.; Anthony, D.M.; Freemantle, N.; Irs, A.; Hill, S.R.; Ward, R.L. Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: A meta-analysis. Lancet Oncol. 2006, 7, 741–746. [Google Scholar] [CrossRef]
- Hotta, K.; Fujiwara, Y.; Matsuo, K.; Kiura, K.; Takigawa, N.; Tabata, M.; Tanimoto, M. Time to Progression as a Surrogate Marker for Overall Survival in Patients with Advanced Non-small Cell Lung Cancer. J. Thorac. Oncol. 2009, 4, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Weir, C.J.; Walley, R.J. Statistical evaluation of biomarkers as surrogate endpoints: A literature review. Stat. Med. 2005, 25, 183–203. [Google Scholar] [CrossRef]
- Fleischer, F.; Gaschler-Markefski, B.; Bluhmki, E. A statistical model for the dependence between progression-free survival and overall survival. Stat. Med. 2009, 28, 2669–2686. [Google Scholar] [CrossRef]
- Hayashi, H.; Okamoto, I.; Taguri, M.; Morita, S.; Nakagawa, K. Postprogression Survival in Patients with Advanced Non–Small-Cell Lung Cancer Who Receive Second-Line or Third-Line Chemotherapy. Clin. Lung Cancer 2013, 14, 261–266. [Google Scholar] [CrossRef]
- Imai, H.; Takahashi, T.; Mori, K.; Ono, A.; Akamatsu, H.; Shukuya, T.; Taira, T.; Kenmotsu, H.; Naito, T.; Murakami, H.; et al. Individual-level data on the relationships of progression-free survival, post-progression survival, and tumor response with overall survival in patients with advanced non-squamous non-small cell lung cancer. Neoplasma 2014, 61, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Mori, K.; Wakuda, K.; Ono, A.; Akamatsu, H.; Shukuya, T.; Taira, T.; Kenmotsu, H.; Naito, T.; Kaira, K.; et al. Progression-free survival, post-progression survival, and tumor response as surrogate markers for overall survival in patients with extensive small cell lung cancer. Ann. Thorac. Med. 2015, 10, 61–66. [Google Scholar] [PubMed]
- Imai, H.; Mori, K.; Ono, A.; Akamatsu, H.; Taira, T.; Kenmotsu, H.; Naito, T.; Kaira, K.; Murakami, H.; Endo, M.; et al. Individual-level data on the relationships of progression-free survival and post-progression survival with overall survival in patients with advanced non-squamous non-small cell lung cancer patients who received second-line chemotherapy. Med. Oncol. 2014, 31, 88. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Mori, K.; Watase, N.; Fujimoto, S.; Kaira, K.; Yamada, M.; Minato, K. Clinical significance of the relationship between progression-free survival or postprogression survival and overall survival in patients with extensive disease-small-cell lung cancer treated with carboplatin plus etoposide. Can. Respir. J. 2016, 2016, 5405810. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).