Blinding in Clinical Trials: Seeing the Big Picture
Abstract
:1. Introduction
2. What Is Blinding?
3. Why Do We Blind?
4. Who and What Do We Blind?
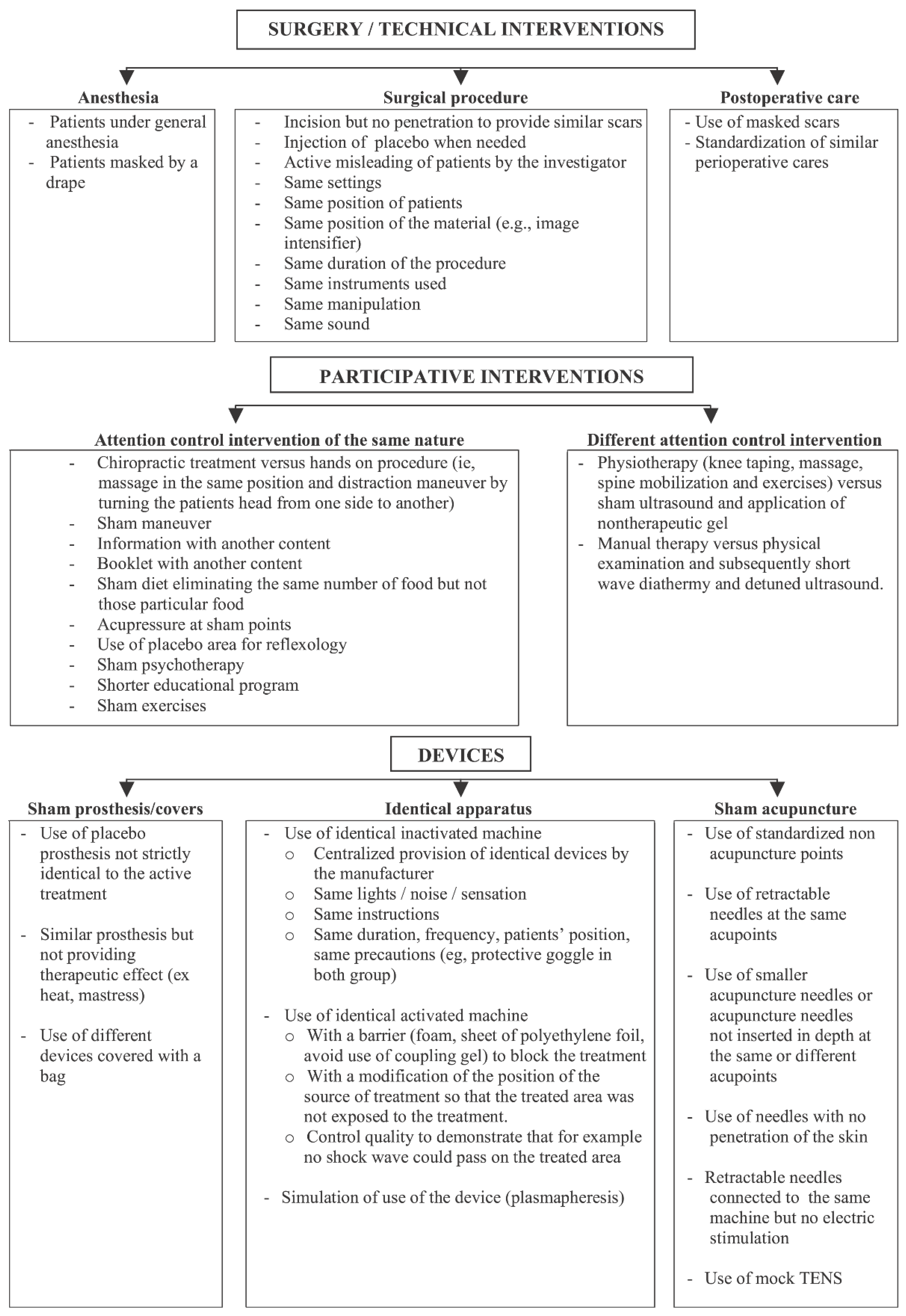
5. How Do We Blind?
6. Limitations of Blinding
7. Blinding: Reporting Responsibly
8. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New evidence pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhide, A.; Shah, P.S.; Acharya, G. A simplified guide to randomized controlled trials. Acta Obstet. Gynecol. Scand. 2018, 97, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Poolman, R.W.; Hanson, B.; Marti, R.K.; Bhandari, M. Conducting a clinical study: A guide for good research practice. Indian J. Orthop. 2007, 41, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Psaty, B.M.; Prentice, R.L. Minimizing bias in randomized trials: The importance of blinding. Jama 2010, 304, 793–794. [Google Scholar] [CrossRef]
- Gresham, G.; Meinert, J.L.; Gresham, A.G.; Meinert, C.L. Assessment of Trends in the Design, Accrual, and Completion of Trials Registered in ClinicalTrials.gov by Sponsor Type, 2000–2019. JAMA Netw. Open 2020, 3, e2014682. [Google Scholar] [CrossRef]
- Catillon, M. Trends and predictors of biomedical research quality, 1990–2015: A meta-research study. BMJ Open 2019, 9, e030342. [Google Scholar] [CrossRef] [Green Version]
- Falagas, M.E.; Pitsouni, E.I.; Bliziotis, I.A. Trends in the methodological quality of published randomized controlled trials on antibacterial agents. Br. J. Clin. Pharmacol. 2008, 65, 942–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahm, P.; N’Dow, J.; Holmberg, L.; Hamdy, F. The Future of Randomised Controlled Trials in Urology. Eur. Urol. 2014, 66, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Djurisic, S.; Rath, A.; Gaber, S.; Garattini, S.; Bertele, V.; Ngwabyt, S.-N.; Hivert, V.; Neugebauer, E.A.M.; Laville, M.; Hiesmayr, M.; et al. Barriers to the conduct of randomised clinical trials within all disease areas. Trials 2017, 18, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakobsen, J.C.; Gluud, C. The necessity of randomized clinical trials. J. Adv. Med. Med. Res. 2013, 3, 1453–1468. [Google Scholar] [CrossRef] [Green Version]
- Kahan, B.C.; Rehal, S.; Cro, S. Blinded Outcome Assessment Was Infrequently Used and Poorly Reported in Open Trials. PLoS ONE 2015, 10, e0131926. [Google Scholar] [CrossRef] [Green Version]
- Karanicolas, P.J.; Bhandari, M.; Taromi, B.; Akl, E.A.; Bassler, D.; Alonso-Coello, P.; Rigau, D.; Bryant, D.; Smith, S.E.; Walter, S.D.; et al. Blinding of Outcomes in Trials of Orthopaedic Trauma: An Opportunity to Enhance the Validity of Clinical Trials. J. Bone Jt. Surg.-Am. Vol. 2008, 90, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Wartolowska, K.; Beard, D.; Carr, A. Blinding in trials of interventional procedures is possible and worthwhile. F1000Research 2017, 6, 1663. [Google Scholar] [CrossRef] [PubMed]
- Akobeng, A.K. Understanding randomised controlled trials. Arch. Dis. Child. 2005, 90, 840–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akobeng, A.K. Principles of evidence based medicine. Arch. Dis. Child. 2005, 90, 837–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novack, L.; Jotkowitz, A.; Knyazer, B.; Novack, V. Evidence-based medicine: Assessment of knowledge of basic epidemiological and research methods among medical doctors. Postgrad. Med. J. 2006, 82, 817–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Day, S.J.; Altman, D.G. Statistics notes: Blinding in clinical trials and other studies. BMJ 2000, 321, 504. [Google Scholar] [CrossRef] [Green Version]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altman, D.G.; Schulz, K.F. Statistics Notes: Concealing treatment allocation in randomised trials. BMJ 2001, 323, 446–447. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.F.; Grimes, D.A. Allocation concealment in randomised trials: Defending against deciphering. Lancet 2002, 359, 614–618. [Google Scholar] [CrossRef]
- Karanicolas, P.J.; Farrokhyar, F.; Bhandari, M. Practical tips for surgical research: Blinding: Who, what, when, why, how? Can. J. Surg. 2010, 53, 345–348. [Google Scholar]
- Pannucci, C.J.; Wilkins, E.G. Identifying and Avoiding Bias in Research. Plast. Reconstr. Surg. 2010, 126, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Grimes, D.A. Blinding in randomised trials: Hiding who got what. Lancet 2002, 359, 696–700. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Pugsley, S.O.; Sullivan, M.J.; Thompson, P.J.; Berman, L.; Jones, N.L.; Fallen, E.L.; Taylor, D.W. Effect of encouragement on walking test performance. Thorax 1984, 39, 818–822. [Google Scholar] [CrossRef] [Green Version]
- Kaptchuk, T.J.; Kelley, J.M.; Conboy, L.A.; Davis, R.B.; Kerr, C.E.; Jacobson, E.E.; Kirsch, I.; Schyner, R.N.; Nam, B.H.; Nguyen, L.T.; et al. Components of placebo effect: Randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008, 336, 999–1003. [Google Scholar] [CrossRef] [Green Version]
- Hróbjartsson, A.; Emanuelsson, F.; Thomsen, A.S.S.; Hilden, J.; Brorson, S. Bias due to lack of patient blinding in clinical trials. A systematic review of trials randomizing patients to blind and nonblind sub-studies. Int. J. Epidemiol. 2014, 43, 1272–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wartolowska, K.; Judge, A.; Hopewell, S.; Collins, G.; Dean, B.J.F.; Rombach, I.; Brindley, D.; Savulescu, J.; Beard, D.J.; Carr, A.J. Use of placebo controls in the evaluation of surgery: Systematic review. BMJ 2014, 348, g3253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hróbjartsson, A.; Thomsen, A.S.S.; Emanuelsson, F.; Tendal, B.; Rasmussen, J.V.; Hilden, J.; Boutron, I.; Ravaud, P.; Brorson, S. Observer bias in randomized clinical trials with time-to-event outcomes: Systematic review of trials with both blinded and non-blinded outcome assessors. Int. J. Epidemiol. 2014, 43, 937–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hróbjartsson, A.; Thomsen, A.S.S.; Emanuelsson, F.; Tendal, B.; Hilden, J.; Boutron, I.; Ravaud, P.; Brorson, S. Observer bias in randomised clinical trials with binary outcomes: Systematic review of trials with both blinded and non-blinded outcome assessors. BMJ 2012, 344, e1119. [Google Scholar] [CrossRef] [Green Version]
- Hróbjartsson, A.; Thomsen, A.S.S.; Emanuelsson, F.; Tendal, B.; Hilden, J.; Boutron, I.; Ravaud, P.; Brorson, S. Observer bias in randomized clinical trials with measurement scale outcomes: A systematic review of trials with both blinded and nonblinded assessors. Can. Med. Assoc. J. 2013, 185, E201–E211. [Google Scholar] [CrossRef] [Green Version]
- United States Food and Drug Administration. Good Review Practice: Clinical Review of Investigational New Drug Applications; FDA: Washington, DC, USA, 2013.
- Page, M.J.; Higgins, J.; Clayton, G.; Sterne, J.; Hróbjartsson, A.; Savović, J. Empirical Evidence of Study Design Biases in Randomized Trials: Systematic Review of Meta-Epidemiological Studies. PLoS ONE 2016, 11, e0159267. [Google Scholar] [CrossRef]
- Moustgaard, H.; Clayton, G.L.; Jones, H.E.; Boutron, I.; Jørgensen, L.; Laursen, D.R.T.; Olsen, M.F.; Paludan-Müller, A.; Ravaud, P.; Savović, J.; et al. Impact of blinding on estimated treatment effects in randomised clinical trials: Meta-epidemiological study. BMJ 2020, 368, l6802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice, 3rd ed.; Pearson; Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Lang, T.A.; Stroup, D.F. Who knew? The misleading specificity of “double-blind” and what to do about it. Trials 2020, 21, 1–7. [Google Scholar] [CrossRef]
- Clifton, L.; Clifton, D.A. How to maintain the maximal level of blinding in randomisation for a placebo-controlled drug trial. Contemp. Clin. Trials Commun. 2019, 14, 100356. [Google Scholar] [CrossRef] [PubMed]
- Karanicolas, P.J.; Bhandari, M.; Walter, S.D.; Heels-Ansdell, D.; Guyatt, G.H. Radiographs of hip fractures were digitally altered to mask surgeons to the type of implant without compromising the reliability of quality ratings or making the rating process more difficult. J. Clin. Epidemiol. 2009, 62, 214–223.e1. [Google Scholar] [CrossRef]
- Boutron, I.; Estellat, C.; Guittet, L.; Dechartres, A.; Sackett, D.L.; Hróbjartsson, A.; Ravaud, P. Methods of Blinding in Reports of Randomized Controlled Trials Assessing Pharmacologic Treatments: A Systematic Review. PLoS Med. 2006, 3, e425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ergina, P.L.; Cook, J.A.; Blazeby, J.M.; Boutron, I.; Clavien, P.A.; Reeves, B.C.; Seiler, C.M.; Altman, D.G.; Aronson, J.K.; Barkun, J.S.; et al. Challenges in evaluating surgical innovation. Lancet 2009, 374, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- McLeod, R.S. Issues in surgical randomized controlled trials. World J. Surg. 1999, 23, 1210–1214. [Google Scholar] [CrossRef]
- Cook, J.A. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.; Egger, M.; Gluud, L.L.; Schulz, K.F.; Jüni, P.; Altman, D.G.; Gluud, C.; Martin, R.M.; Wood, A.J.G.; Sterne, J.A.C. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: Meta-epidemiological study. BMJ 2008, 336, 601–605. [Google Scholar] [CrossRef] [Green Version]
- Boutron, I.; Guittet, L.; Estellat, C.; Moher, D.; Hróbjartsson, A.; Ravaud, P. Reporting Methods of Blinding in Randomized Trials Assessing Nonpharmacological Treatments. PLoS Med. 2007, 4, e61. [Google Scholar] [CrossRef]
- Freeman, B.J.; Fraser, R.D.; Cain, C.M.; Hall, D.J.; Chapple, D.C. A randomized, double-blind, controlled trial: Intradiscal electrothermal therapy versus placebo for the treatment of chronic discogenic low back pain. Spine 2005, 30, 2369–2377, discussion 2378. [Google Scholar] [CrossRef]
- Gillespie, M.B.; Wylie, P.E.; Lee-Chiong, T.; Rapoport, D.M. Effect of Palatal Implants on Continuous Positive Airway Pressure and Compliance. Otolaryngol. Neck Surg. 2010, 144, 230–236. [Google Scholar] [CrossRef]
- Cotton, P.B.; Durkalski, V.; Romagnuolo, J.; Pauls, Q.; Fogel, E.; Tarnasky, P.; Aliperti, G.; Freeman, M.; Kozarek, R.; Jamidar, P.; et al. Effect of endoscopic sphincterotomy for suspected sphincter of Oddi dysfunction on pain-related disability following cholecystectomy: The EPISOD randomized clinical trial. Jama 2014, 311, 2101–2109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, M.; Orlu-Gul, M.; Legay, H.; Tuleu, C. Blinding in pharmacological trials: The devil is in the details. Arch. Dis. Child. 2013, 98, 656–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avenell, A.; Grant, A.M.; McGee, M.; McPherson, G.; Campbell, M.K.; McGee, M.A.; RECORD Trial Management Group. The effects of an open design on trial participant recruitment, compliance and retention—A randomized controlled trial comparison with a blinded, placebo-controlled design. Clin. Trials 2004, 1, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Hemminki, E.; Hovi, S.-L.; Veerus, P.; Sevón, T.; Tuimala, R.; Rahu, M.; Hakama, M. Blinding decreased recruitment in a prevention trial of postmenopausal hormone therapy. J. Clin. Epidemiol. 2004, 57, 1237–1243. [Google Scholar] [CrossRef]
- Treweek, S.; Pitkethly, M.; Cook, J.; Fraser, C.; Mitchell, E.; Sullivan, F.; Jackson, C.; Taskila, T.K.; Gardner, H. Strategies to improve recruitment to randomised trials. Cochrane Database Syst. Rev. 2018, 2, Mr000013. [Google Scholar] [CrossRef] [Green Version]
- Treweek, S.; Zwarenstein, M. Making trials matter: Pragmatic and explanatory trials and the problem of applicability. Trials 2009, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Blader, J.C. Can keeping clinical trial participants blind to their study treatment adversely affect subsequent care? Contemp. Clin. Trials 2005, 26, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Anand, R.; Norrie, J.; Bradley, J.M.; McAuley, D.F.; Clarke, M. Fool’s gold? Why blinded trials are not always best. BMJ 2020, 368, l6228. [Google Scholar] [CrossRef] [Green Version]
- Kemmler, G.; Hummer, M.; Widschwendter, C.; Fleischhacker, W.W. Dropout rates in placebo-controlled and active-control clinical trials of antipsychotic drugs: A meta-analysis. Arch. Gen. Psychiatry 2005, 62, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.F. Subverting randomization in controlled trials. JAMA 1995, 274, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Tempini, N.; Teira, D. Is the genie out of the bottle? Digital platforms and the future of clinical trials. Econ. Soc. 2019, 48, 77–106. [Google Scholar] [CrossRef] [Green Version]
- Wicks, P.; Vaughan, T.; Heywood, J. Subjects no more: What happens when trial participants realize they hold the power? BMJ 2014, 348, g368. [Google Scholar] [CrossRef] [Green Version]
- Feys, F.; Bekkering, G.E.; Singh, K.; Devroey, D. Do randomized clinical trials with inadequate blinding report enhanced placebo effects for intervention groups and nocebo effects for placebo groups? Syst. Rev. 2014, 3, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quittell, L.M. The Scientific and Social Implications of Unblinding a Study Subject. Am. J. Bioeth. 2018, 18, 71–73. [Google Scholar] [CrossRef]
- Cook, T.D.; Campbell, D.T.; Day, A. Quasi-Experimentation: Design & Analysis Issues for Field Settings; Houghton Mifflin: Boston, MA, USA, 1979; Volume 351. [Google Scholar]
- Onghena, P. Resentful Demoralization. In Encyclopedia of Statistics in Behavioral Science; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Sackett, D.L. Commentary: Measuring the success of blinding in RCTs: Don’t, must, can’t or needn’t? Int. J. Epidemiol. 2007, 36, 664–665. [Google Scholar] [CrossRef] [Green Version]
- Bang, H.; Flaherty, S.P.; Kolahi, J.; Park, J. Blinding assessment in clinical trials: A review of statistical methods and a proposal of blinding assessment protocol. Clin. Res. Regul. Aff. 2010, 27, 42–51. [Google Scholar] [CrossRef]
- Bang, H.; Ni, L.; Davis, C.E. Assessment of blinding in clinical trials. Control. Clin. Trials 2004, 25, 143–156. [Google Scholar] [CrossRef]
- James, K.E.; Bloch, D.A.; Lee, K.K.; Kraemer, H.C.; Fuller, R.K. An index for assessing blindness in a multi-centre clinical trial: Disulfiram for alcohol cessation-a VA cooperative study. Stat. Med. 1996, 15, 1421–1434. [Google Scholar] [CrossRef]
- Boutron, I.; Estellat, C.; Ravaud, P. A review of blinding in randomized controlled trials found results inconsistent and questionable. J. Clin. Epidemiol. 2005, 58, 1220–1226. [Google Scholar] [CrossRef]
- Fergusson, D.; Glass, K.C.; Waring, D.; Shapiro, S. Turning a blind eye: The success of blinding reported in a random sample of randomised, placebo controlled trials. BMJ 2004, 328, 432. [Google Scholar] [CrossRef] [Green Version]
- Hrobjartsson, A.; Forfang, E.; Haahr, M.T.; Als-Nielsen, B.; Brorson, S. Blinded trials taken to the test: An analysis of randomized clinical trials that report tests for the success of blinding. Int. J. Epidemiol. 2007, 36, 654–663. [Google Scholar] [CrossRef]
- Hemilä, H. Assessment of blinding may be inappropriate after the trial. Contemp. Clin. Trials 2005, 26, 512–514. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, E.; Herbert, R.D.; McGeechan, K.; Herbert, J.J.; Barratt, A.L. A theoretical analysis showed that blinding cannot eliminate potential for bias associated with beliefs about allocation in randomized clinical trials. J. Clin. Epidemiol. 2014, 67, 667–671. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devereaux, P.J.; Manns, B.J.; Ghali, W.A.; Quan, H.; Lacchetti, C.; Montori, V.M.; Bhandari, M.; Guyatt, G.H. Physician interpretations and textbook definitions of blinding terminology in randomized controlled trials. JAMA 2001, 285, 2000–2003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, K.F.; Chalmers, I.; Altman, U.G. The landscape and lexicon of blinding in randomized trials. Ann. Intern. Med. 2002, 136, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Haahr, M.T.; Hróbjartsson, A. Who is blinded in randomized clinical trials? A study of 200 trials and a survey of authors. Clin. Trials 2006, 3, 360–365. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D.; The Consort Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Bello, S.; Moustgaard, H.; Hróbjartsson, A. The risk of unblinding was infrequently and incompletely reported in 300 randomized clinical trial publications. J. Clin. Epidemiol. 2014, 67, 1059–1069. [Google Scholar] [CrossRef]
- Penić, A.; Begić, D.; Balajić, K.; Kowalski, M.; Marušić, A.; Puljak, L. Definitions of blinding in randomised controlled trials of interventions published in high-impact anaesthesiology journals: A methodological study and survey of authors. BMJ Open 2020, 10, e035168. [Google Scholar] [CrossRef] [Green Version]
- Probst, P.; Zaschke, S.; Heger, P.; Harnoss, J.C.; Hüttner, F.J.; Mihaljevic, A.L.; Knebel, P.; Diener, M.K. Evidence-based recommendations for blinding in surgical trials. Langenbeck’s Arch. Surg. 2019, 404, 273–284. [Google Scholar] [CrossRef]
- Sackett, D.L. Turning a blind eye: Why we don’t test for blindness at the end of our trials. BMJ 2004, 328, 1136. [Google Scholar] [CrossRef] [Green Version]
- Castro, M. Placebo versus Best-Available-Therapy Control Group in Clinical Trials for Pharmacologic Therapies: Which Is Better? Proc. Am. Thorac. Soc. 2007, 4, 570–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olfson, M.; Marcus, S.C. Decline in Placebo-Controlled Trial Results Suggests New Directions For Comparative Effectiveness Research. Health Aff. 2013, 32, 1116–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, A.C. Implications of Clinical Trial Design on Sample Size Requirements. Schizophr. Bull. 2007, 34, 664–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parasrampuria, D.A.; Benet, L.Z. Inclusion of Placebos and Blinding for Ascending Dose First-in-Human Studies and Other Underpowered Phase 1 Studies has not been Justified and on Balance is Not Useful. Basic Clin. Pharmacol. Toxicol. 2014, 117, 44–51. [Google Scholar] [CrossRef] [Green Version]
- United States Food and Drug Administration. 22 Case Studies Where Phase 2 and Phase 3 Trials Had Divergent Results; FDA: Washington, DC, USA, 2017.
- Friede, T.; Kieser, M. Blinded sample size re-estimation in superiority and noninferiority trials: Bias versus variance in variance estimation. Pharm. Stat. 2013, 12, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Friede, T.; Pohlmann, H.; Schmidli, H. Blinded sample size reestimation in event-driven clinical trials: Methods and an application in multiple sclerosis. Pharm. Stat. 2019, 18, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Friede, T.; Schmidli, H. Blinded sample size reestimation with count data: Methods and applications in multiple sclerosis. Stat. Med. 2010, 29, 1145–1156. [Google Scholar] [CrossRef] [PubMed]

| Group or Individual Blinded a | Information Withheld b | Method of Blinding c,d | Blinding Compromised |
|---|---|---|---|
| Required fields to be completed for all trials described as blinded | |||
| Person assigning participants to groups | Group assignment | Concealed allocation schedule | No |
| Participants | Group assignment | Placebo medications; sham surgeries | No |
| Care providers | Group assignment | Not told of group assignment | No |
| Data collectors and managers | Group assignment | Not told of group assignment | No |
| Outcome assessors | Purpose of study; group assignment; participant characteristics | Participants given numerical identifiers | No |
| Statisticians | Participant and group identities | Participants and groups given numerical identifiers | No |
| Supplemental fields for all blinded groups or individuals not mentioned above | |||
| Trial manager | Not applicable | ... | ... |
| Pharmacists | Not applicable | ... | ... |
| Laboratory technicians | Participant identities | Participants given numerical identifiers | |
| Outcome adjudicators | Group assignment | Groups given numerical identifiers | Yes (put details in text) |
| Data monitoring and safety committees | Not applicable | ... | ... |
| Manuscript writers | Not blinded | ... | ... |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaghan, T.F.; Agudelo, C.W.; Rahman, S.N.; Wein, A.J.; Lazar, J.M.; Everaert, K.; Dmochowski, R.R. Blinding in Clinical Trials: Seeing the Big Picture. Medicina 2021, 57, 647. https://doi.org/10.3390/medicina57070647
Monaghan TF, Agudelo CW, Rahman SN, Wein AJ, Lazar JM, Everaert K, Dmochowski RR. Blinding in Clinical Trials: Seeing the Big Picture. Medicina. 2021; 57(7):647. https://doi.org/10.3390/medicina57070647
Chicago/Turabian StyleMonaghan, Thomas F., Christina W. Agudelo, Syed N. Rahman, Alan J. Wein, Jason M. Lazar, Karel Everaert, and Roger R. Dmochowski. 2021. "Blinding in Clinical Trials: Seeing the Big Picture" Medicina 57, no. 7: 647. https://doi.org/10.3390/medicina57070647
APA StyleMonaghan, T. F., Agudelo, C. W., Rahman, S. N., Wein, A. J., Lazar, J. M., Everaert, K., & Dmochowski, R. R. (2021). Blinding in Clinical Trials: Seeing the Big Picture. Medicina, 57(7), 647. https://doi.org/10.3390/medicina57070647





