Long-Term Effects of Renal Artery Denervation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Collection
2.5. Renal Artery Sympathetic Denervation
2.6. Arterial Stiffness and Wave Reflection Measurements
2.7. Follow-Up
2.8. Statistical Analysis
3. Results
3.1. Study Population
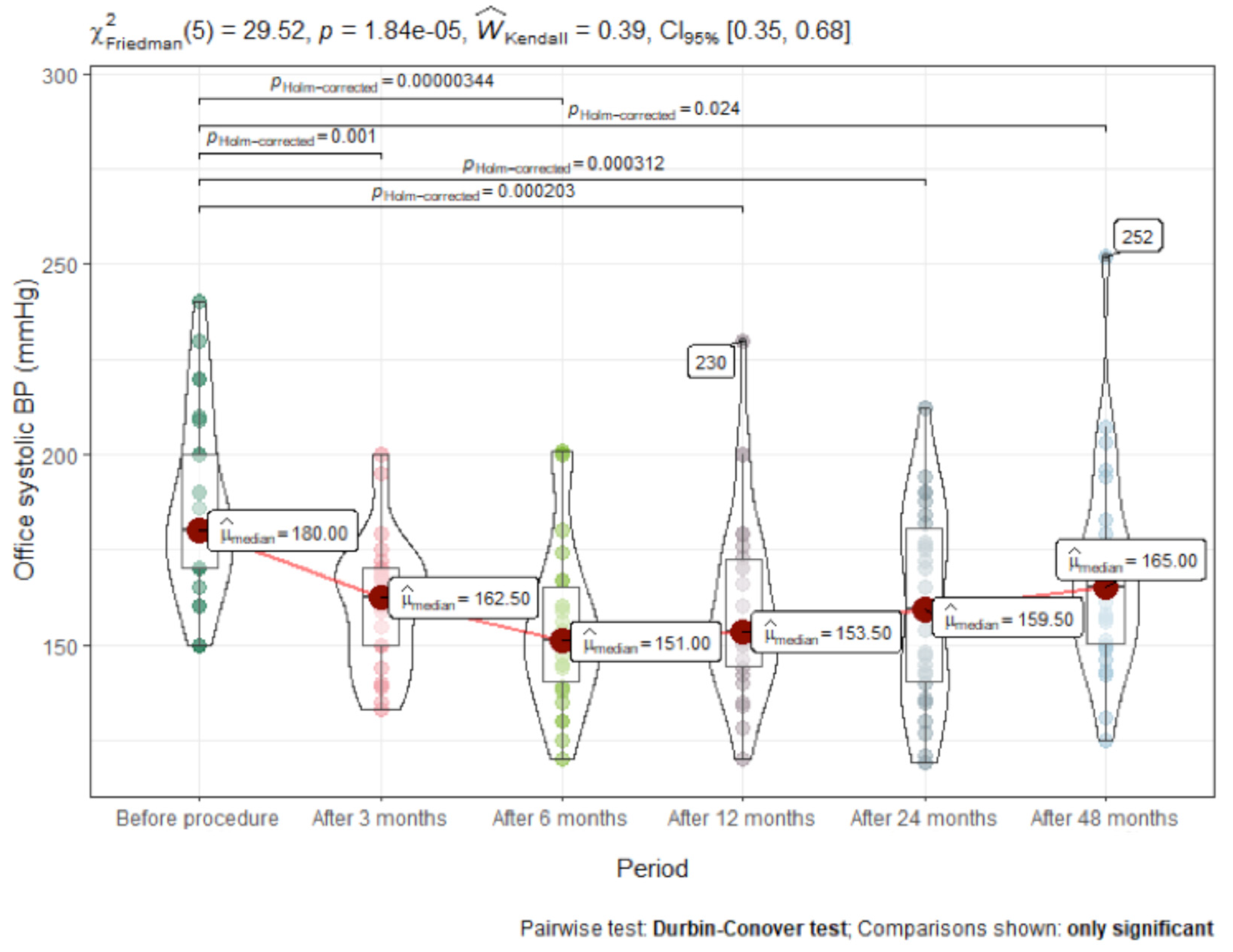
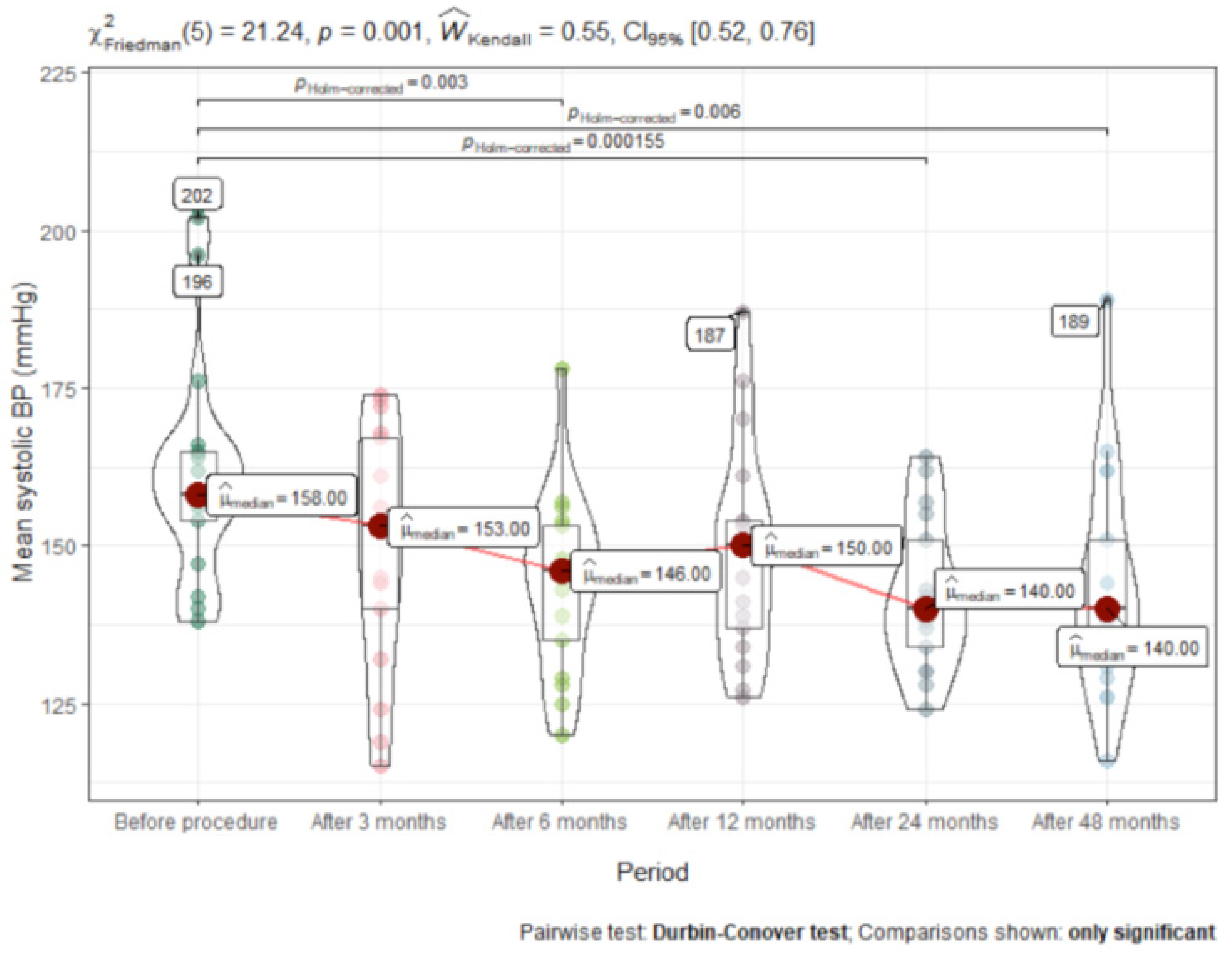
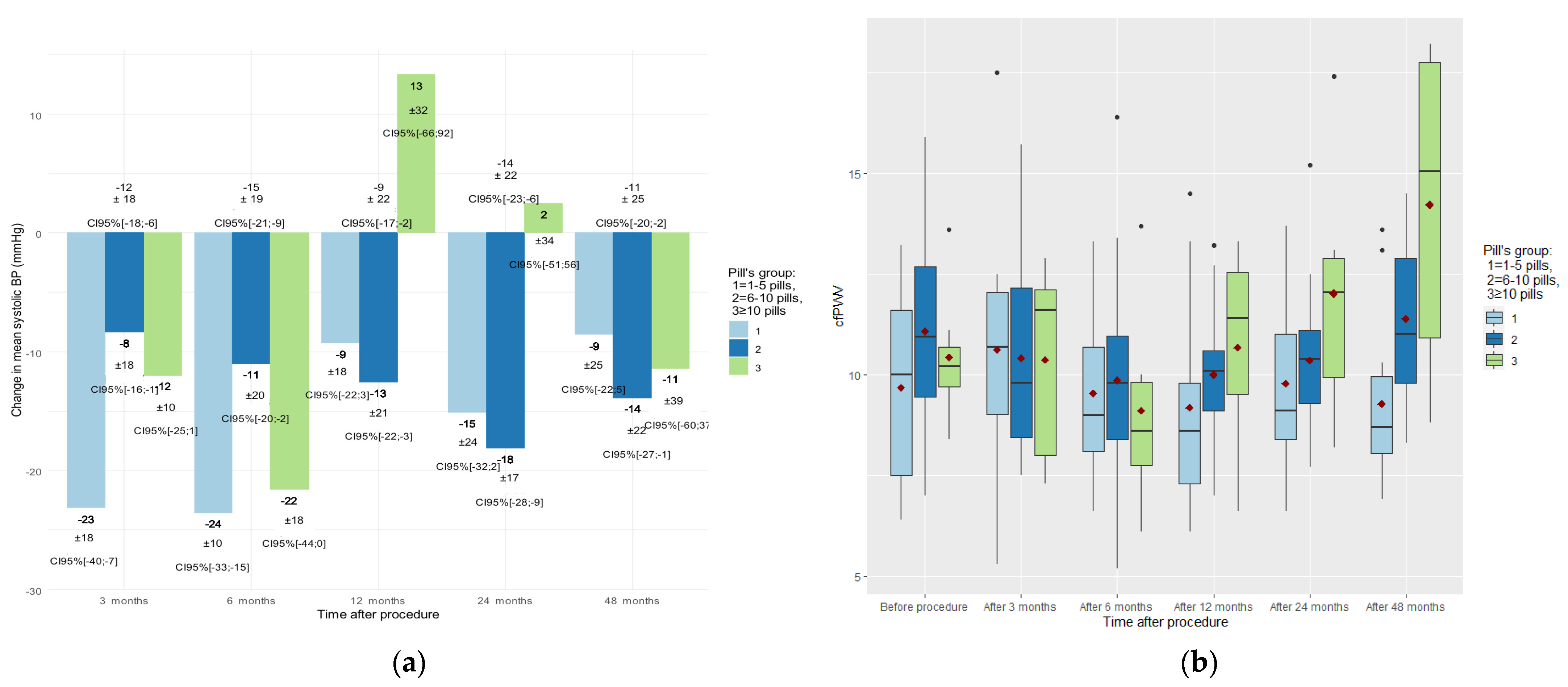
3.2. Time Course of Change in Blood Pressure
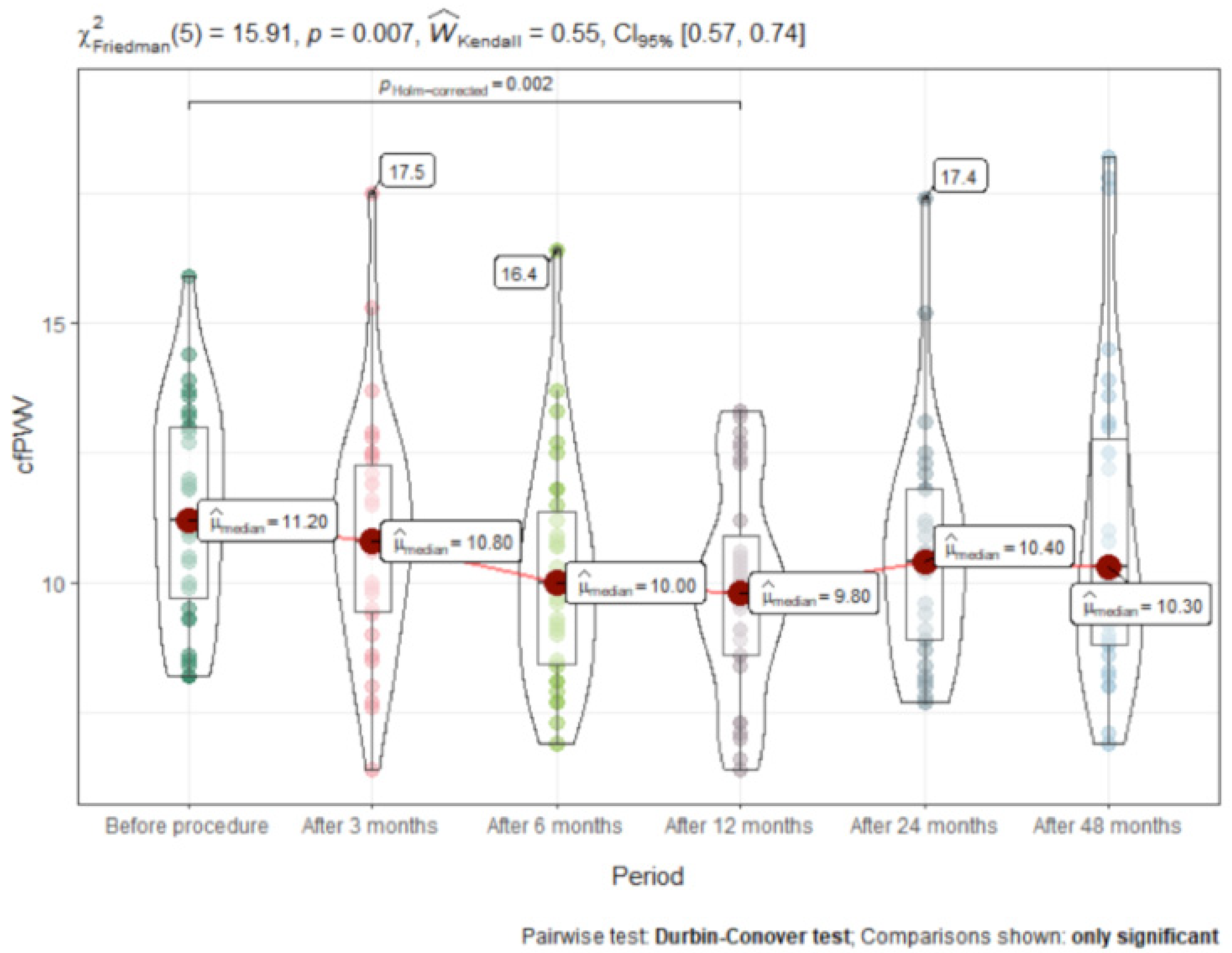
3.3. Time Course of Change in Carotid-Femoral Pulse Wave Velocity (cfPWV)
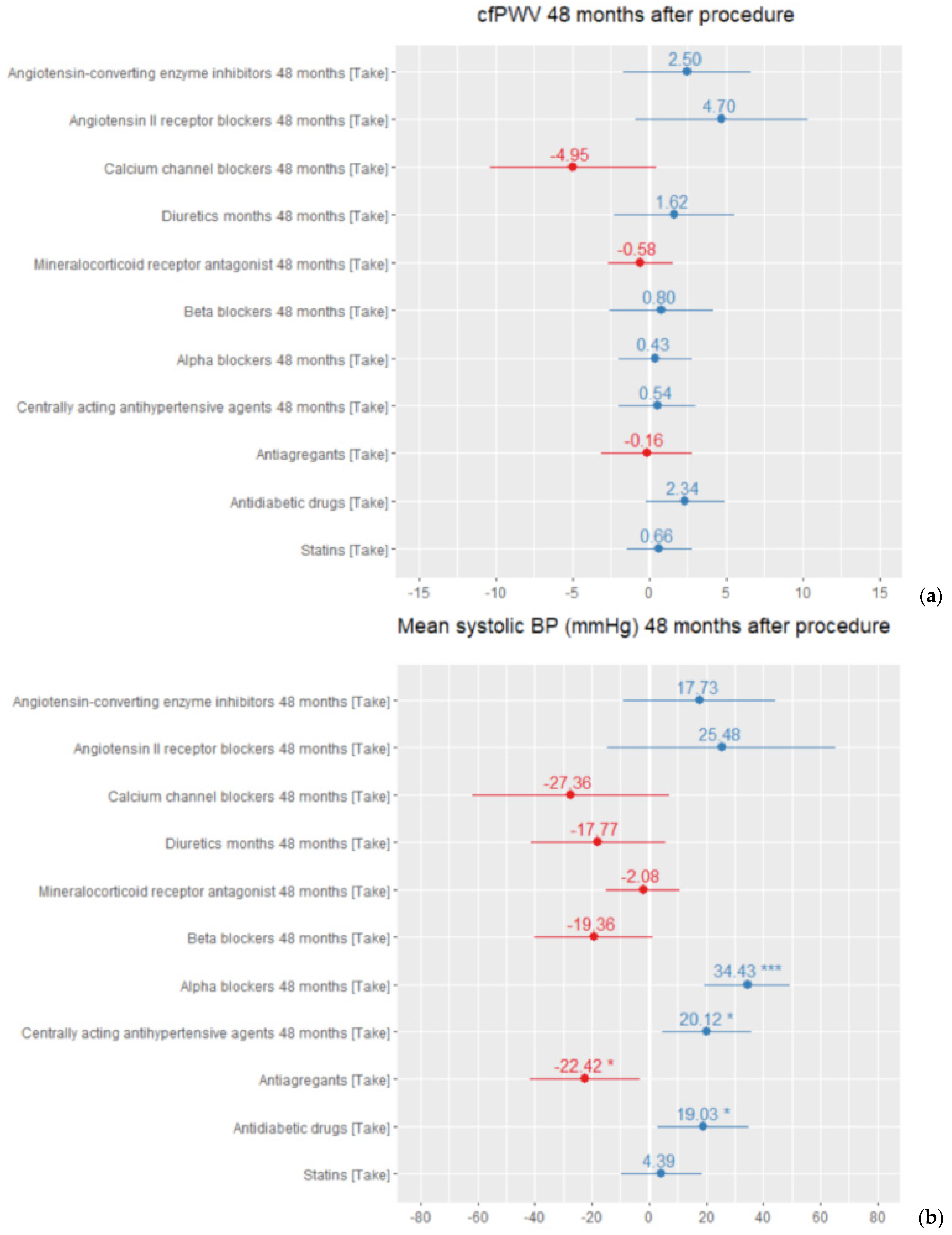
4. Discussion
5. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0140673616316798 (accessed on 25 May 2020). [CrossRef] [Green Version]
- Gupta, A.; Prince, M.; Bob-Manuel, T.; Jenkins, J.S. Renal denervation: Alternative treatment options for hypertension? Prog. Cardiovasc. Dis. 2020, 63, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.S.; Burt, V.; Louis, T.; Carroll, M.D. Hypertension Among Adults in the United States, 2009–2010; National Center for Health Statistics: Hyattsville, MD, USA, 2012; pp. 1–8.
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Himmelfarb, C.D.; Egan, B.M.; Flack, J.M.; Gidding, S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef]
- Bourque, G.; Ilin, J.V.; Ruzicka, M.; Davis, A.; Hiremath, S. The Prevalence of Nonadherence in Patients With Resistant Hypertension: A Systematic Review Protocol. Can. J. Kidney Health Dis. 2019, 6. [Google Scholar] [CrossRef]
- Kandzari, D.E.; Bhatt, D.L.; Sobotka, P.A.; O’Neill, W.W.; Esler, M.; Flack, J.M.; Katzen, B.T.; Leon, M.B.; Massaro, J.M.; Negoita, M.; et al. Catheter-Based Renal Denervation for Resistant Hypertension: Rationale and Design of the SYMPLICITY HTN-3 Trial. Clin. Cardiol. 2012, 35, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, D.E.; Kario, K.; Mahfoud, F.; Cohen, S.A.; Pilcher, G.; Pocock, S.; Townsend, R.; Weber, M.A.; Böhm, M. The SPYRAL HTN Global Clinical Trial Program: Rationale and design for studies of renal denervation in the absence (SPYRAL HTN OFF-MED) and presence (SPYRAL HTN ON-MED) of antihypertensive medications. Am. Hear. J. 2016, 171, 82–91. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0002870315005803 (accessed on 25 May 2020). [CrossRef]
- Pancholy, S.B.; Shantha, G.P.S.; Patel, T.M.; Sobotka, P.A.; Kandzari, D.E. Meta-Analysis of the Effect of Renal Denervation on Blood Pressure and Pulse Pressure in Patients With Resistant Systemic Hypertension. Am. J. Cardiol. 2014, 114, 856–861. [Google Scholar] [CrossRef]
- Van Bortel, L.M.; Laurent, S.; Boutouyrie, P.; Chowienczyk, P.; Cruickshank, J.K.; De Backer, T.; Filipovsky, J.; Huybrechts, S.; Mattace-Raso, F.U.; Protogerou, A.D.; et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J. Hypertens. 2012, 30, 445–448. Available online: https://journals.lww.com/00004872-201203000-00001 (accessed on 16 March 2020). [CrossRef] [PubMed] [Green Version]
- Safar, M.E.; Thomas, F.; Blacher, J.; Nzietchueng, R.; Bureau, J.-M.; Pannier, B.; Benetos, A. Metabolic Syndrome and Age-Related Progression of Aortic Stiffness. J. Am. Coll. Cardiol. 2006, 47, 72–75. [Google Scholar] [CrossRef]
- Brandt, M.C.; Reda, S.; Mahfoud, F.; Lenski, M.; Böhm, M.; Hoppe, U.C. Effects of Renal Sympathetic Denervation on Arterial Stiffness and Central Hemodynamics in Patients With Resistant Hypertension. J. Am. Coll. Cardiol. 2012, 60, 1956–1965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berukstis, A.; Navickas, R.; Neverauskaite-Piliponiene, G.; Ryliskyte, L.; Misiura, J.; Vajauskas, D.; Misonis, N.; Laucevicius, A. Arterial Destiffening Starts Early after Renal Artery Denervation. Int. J. Hypertens. 2019, 2019, 3845690. [Google Scholar] [CrossRef] [PubMed]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Burnier, M.; Egan, B.M. Adherence in Hypertension: A Review of Prevalence, Risk Factors, Impact, and Management. Circ. Res. 2019, 124, 1124–1140. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease; Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013, 3, 5–14. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1474667016423049 (accessed on 12 June 2021).
- Mahfoud, F.; Böhm, M.; Schmieder, R.; Narkiewicz, K.; Ewen, S.; Ruilope, L.; Schlaich, M.; Williams, B.; Fahy, M.; Mancia, G. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur. Hear. J. 2019, 40, 3474–3482. [Google Scholar] [CrossRef]
- Lauder, L.; Böhm, M.; Mahfoud, F. Where is renal nerve ablation going? Eur. Hear. J. 2020, 41, 4538–4540. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, D.; Luo, S.; Qin, S. Effect of Catheter-Based Renal Denervation on Uncontrolled Hypertension: A Systematic Review and Meta-analysis. Mayo Clin. Proc. 2019, 94, 1695–1706. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Laurent, S. Mechanisms of Arterial Stiffening: From Mechanotransduction to Epigenetics. Arterioscler. Thromb. Vasc. Biol. 2020, ATVBAHA119313129. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32075419 (accessed on 6 February 2021). [CrossRef]
- AlSharari, R.; Lip, G.Y.H.; Shantsila, A. Assessment of Arterial Stiffness in Patients With Resistant Hypertension: Additional Insights Into the Pathophysiology of This Condition? Am. J. Hypertens. 2019, 33, 107–115. [Google Scholar] [CrossRef]
- Elia, L.; Kunderfranco, P.; Carullo, P.; Vacchiano, M.; Farina, F.M.; Hall, I.F.; Mantero, S.; Panico, C.; Papait, R.; Condorelli, G.; et al. UHRF1 epigenetically orchestrates smooth muscle cell plasticity in arterial disease. J. Clin. Investig. 2018, 128, 2473–2486. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29558369 (accessed on 8 March 2020). [CrossRef] [PubMed]
- Diaz, A.; Tringler, M.; Wray, S.; Ramirez, A.J.; Cabrera Fischer, E.I. The effects of age on pulse wave velocity in untreated hypertension. Nephrol. Dial Transplant. 2019, 20, 258–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ott, C.; Franzen, K.F.; Graf, T.; Weil, J.; Schmieder, R.E.; Reppel, M.; Mortensen, K. Renal denervation improves 24-hour central and peripheral blood pressures, arterial stiffness, and peripheral resistance. J. Clin. Hypertens. 2018, 20, 366–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Values (Mean (SD) [Min, Max]) | |
|---|---|---|
| Age, years | 56.0 (7.76) [33.0, 72.0] | |
| Sex | ||
| Male | n = 34 (46.5%) | |
| Female | n = 39 (53.5%) | |
| Waist circumference, cm | 115.5 (10.7) [95, 129] | |
| Height, cm | 168.6 (8.6) [150, 186] | |
| Weight, kg | 97.6 (17.7) [60.0, 134] | |
| BMI (kg/m2) | 34.3 (5.6) [22.1, 51.2] | |
| Drug regimen | Before procedure | 48 months after procedure |
| Number of antihypertensive drugs | 5.97 (1.1) [4–11] | 5.45 (2.2) [3–11] |
| Total number of pills | 7.33 (2.44) [2–14] | 6.92 (3.37) [2–16] |
| Prescribed drug classes | ||
| ACE-I/ARB | 73 (100%) | 47 (95.9%) |
| Diuretics | 67 (91.8%) | 39 (79.6%) |
| Calcium channel blockers | 63 (86.3%) | 42 (85.7%) |
| Beta blockers | 66 (90.4%) | 27 (55.1%) |
| Mineral receptor antagonists | 48 (65.7%) | 43 (87.8%) |
| Centrally acting agents | 57 (78.1%) | 24 (49.0%) |
| Alpha blockers | 45 (61.6%) | 32 (65.3%) |
| Oral antidiabetic drugs * | 29 (39.7%) | 18 (36,7%) |
| Oral anticoagulants | 5 (6.8%) | 8 (16.3%) |
| Antiplatelet drugs | 21 (28.7%) | 23 (46.9%) |
| Statins | 32 (44.4%) | 24 (48%) |
| Variable (Median IQR) | Before Procedure | After 3 months | After 6 months | After 12 months | After 24 months | After 48 months | p-Value | Effect Size |
|---|---|---|---|---|---|---|---|---|
| Office systolic BP | 180 (40.0) | 162.5 (26.2) | 151 (29.0) | 153 (32.0) | 169 (38.5) | 165 (34.0) | <0.001 | 0.17 |
| Office diastolic BP | 110 (15.0) | 94.5 (16.2) | 89.5 (18.5) | 93.5 (17.0) | 95 (21.5) | 95 (16.5) | <0.001 | 0.12 |
| Office heart rate | 68 (8.2) | 64 (10.0) | 69 (10.5) | 72 (15.0) | 63 (22.5) | 74 (14.8) | 0.052 | 0.03 |
| Mean systolic BP | 158 (23.5) | 153 (31.0) | 146 (26.0) | 150 (25.0) | 140.5 (20.8) | 140 (26.5) | <0.001 | 0.07 |
| Mean diastolic BP | 100 (14.2) | 92 (19.0) | 89 (13.0) | 92 (15.2) | 90.5 (14.8) | 86 (16.2) | 0.01 | 0.04 |
| Mean heart rate | 71.5 (12.8) | 68 (11.0) | 67 (14.5) | 66 (11.0) | 65.5 (13.5) | 70 (11.5) | NS | 0.01 |
| Daytime mean systolic BP | 160 (23.5) | 153 (28.0) | 150 (26.0) | 154 (27.8) | 145 (18.8) | 143 (22.8) | <0.001 | 0.08 |
| Daytime mean diastolic BP | 104 (16.5) | 94 (18.0) | 94 (13.5) | 93 (18.0) | 92 (15.0) | 98 (14.8) | 0.001 | 0.05 |
| Daytime mean heart rate | 75.0 (13.0) | 70.5 (11.2) | 71.0 (15.0) | 70.0 (12.5) | 70.0 (14.5) | 71.5 (14.5) | NS | 0.01 |
| Night-time mean systolic BP | 154 (29.0) | 141 (25.0) | 131 (28.8) | 141 (27.0) | 130 (37.2) | 133 (40.2) | 0.014 | 0.05 |
| Night-time mean diastolic BP | 91 (17.5) | 84 (19.0) | 82 (12.8) | 84 (15.2) | 81 (18.0) | 77 (17.0) | NS | 0.02 |
| Night-time mean heart rate | 64.0 (11.8) | 62.0 (11.2) | 60.0 (9.8) | 60.0 (8.5) | 61.0 (14.8) | 61.5 (13.2) | NS | 0.01 |
| Systolic BP dipping | 7.1 (13.0) | 5.5 (10.3) | 7.1 (10.3) | 7.5 (7.3) | 6.4 (11.6) | 8.6 (13.0) | NS | 0.00 |
| Diastolic BP dipping | 10.2 (11.9) | 9.6 (12.4) | 11.9 (8.5) | 11.7 (9.7) | 8.4 (8.8) | 11.6 (11.9) | NS | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juknevičius, V.; Berūkštis, A.; Juknevičienė, R.; Jasiūnas, E.; Šerpytis, P.; Laucevičius, A. Long-Term Effects of Renal Artery Denervation. Medicina 2021, 57, 662. https://doi.org/10.3390/medicina57070662
Juknevičius V, Berūkštis A, Juknevičienė R, Jasiūnas E, Šerpytis P, Laucevičius A. Long-Term Effects of Renal Artery Denervation. Medicina. 2021; 57(7):662. https://doi.org/10.3390/medicina57070662
Chicago/Turabian StyleJuknevičius, Vytautas, Andrius Berūkštis, Renata Juknevičienė, Eugenijus Jasiūnas, Pranas Šerpytis, and Aleksandras Laucevičius. 2021. "Long-Term Effects of Renal Artery Denervation" Medicina 57, no. 7: 662. https://doi.org/10.3390/medicina57070662
APA StyleJuknevičius, V., Berūkštis, A., Juknevičienė, R., Jasiūnas, E., Šerpytis, P., & Laucevičius, A. (2021). Long-Term Effects of Renal Artery Denervation. Medicina, 57(7), 662. https://doi.org/10.3390/medicina57070662







