Continuous Intravenous Administration of Granulocyte-Colony-Stimulating Factors—A Breakthrough in the Treatment of Cancer Patients with Febrile Neutropenia
Abstract
:1. Introduction
2. Materials and Methods
- Age ≥ 18 years;
- Patients presenting histologically with confirmed solid tumor or hemopathy;
- Patients being treated with chemotherapy (regardless of the cycle);
- Patients who were prescribed treatment with G-CSF as adjunctive therapy for neutropenia (prophylaxis with G-CSSF was allowed);
- Fever is defined per institutional protocol as an oral or axillary temperature above 38 °C, with a presumed infectious etiology (even non-documented by positive bacteriological cultures) in the absence of paraneoplastic or non-infectious causes, for example, blood transfusion;
- Neutropenia (granulocyte count < 500/mm3) induced by curative or palliative chemotherapy regimens, without a cause of bone marrow failure;
- Treatment as an inpatient, with antibiotic regimen (per institutional protocols).
- The exclusion criteria:
- Patients with prior chronic or acute antibiotic therapy for a bacterial infection;
- Patients with shock (whatever the etiology) (systolic blood pressure less than 90 mm Hg, less responsive to treatment peripheral perfusion, and coma or altered mental status);
- Patients subject to a bone marrow transplantation procedure;
- Patients with severe renal failure or impairment (creatinine clearance rate < 15 mL/min/1.73 m2 surface body);
- Patients with abnormal liver function (transaminases elevated more than five times compared with the upper limit of normal or bilirubin more than 3 mg/dL);
- Patients with pregnancy positive test or breast-feeding;
- Patients allergic to antibiotics or any of the ingredients of G-CSF product;
- Patients presenting with a myelodysplastic syndrome;
- Patients not treated with chemotherapy;
- Patients that were included and excluded from a clinical trial less than 90 days from the actual study.
- Shortening the recovery time from febrile neutropenia.
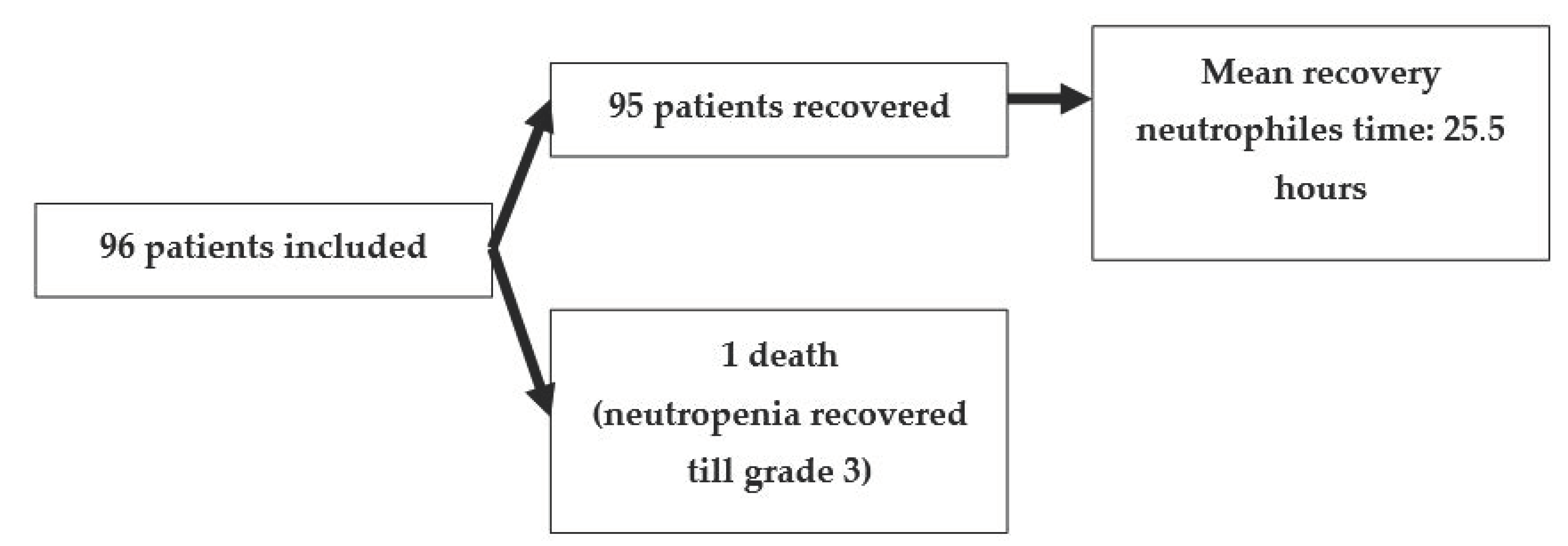
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [Green Version]
- Long, B.; Koyfman, A. Oncologic Emergencies: The Fever with too Few Neutrophils. J. Emerg. Med. 2019, 57, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Klastersky, J.; de Naurois, J.; Rolston, K.; Rapoport, B.; Maschmeyer, G.; Aapro, M.; Herrstedt, J. Management of febrile neutropaenia: ESMO clinical practice guidelines. Ann. Oncol. 2016, 27, 111–118. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0923753419316436 (accessed on 1 April 2021). [CrossRef]
- Becker, P.S.; Griffiths, E.A.; Alwan, L.M.; Bachiashvili, K.; Brown, A.; Cool, R.; Curtin, P.; Dinner, S.; Gojo, I.; Hicks, A.; et al. NCCN Guidelines Insights: Hematopoietic Growth Factors, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 12–22. Available online: https://jnccn.org/view/journals/jnccn/18/1/article-p12.xml (accessed on 1 April 2021). [CrossRef] [Green Version]
- Krzyzanski, W.; Wiczling, P.; Lowe, P.; Pigeolet, E.; Fink, M.; Berghout, A.; Balser, S. Population modeling of filgrastim PK-PD in healthy adults following intravenous and subcutaneous administrations. J. Clin. Pharmacol. 2010, 50, 817–826. [Google Scholar] [CrossRef]
- Bronchud, M.H.; Potter, M.R.; Morgenstern, G.; Blasco, M.J.; Scarffe, J.H.; Thatcher, N.; Crowther, D.; Souza, L.M.; Alton, N.K.; Testa, N.G. In vitro and in vivo analysis of the effects of recombinant human granulocyte colony-stimulating factor in patients. Br. J. Cancer 1988, 58, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Shah, B.; Burg, N.; Pillinger, M.H. Neutrophils, 10th ed.; Kelley and Firestein’s Textbook of Rheumatology, 2-Volume Set; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 169–188.e3. [Google Scholar] [CrossRef]
- Foley, C.; Mackey, M.C. Mathematical model for G-CSF administration after chemotherapy. J. Theor. Biol. 2009, 257, 27–44. [Google Scholar] [CrossRef]
- Campa, C.C.; Germena, G.; Ciraolo, E.; Copperi, F.; Sapienza, A.; Franco, I.; Ghigo, A.; Camporele, A.; DI Savino, A.; Martini, M.; et al. Rac signal adaptation controls neutrophil mobilization from the bone marrow. Sci. Signal. 2016, 9. Available online: https://pubmed.ncbi.nlm.nih.gov/27999173/ (accessed on 1 April 2021).
- Campa, C.C.; Hirsch, E. Rab11 and phosphoinositides: A synergy of signal transducers in the control of vesicular trafficking. In Advances in Biological Regulation; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 63, pp. 132–139. Available online: https://pubmed.ncbi.nlm.nih.gov/27658318/ (accessed on 1 April 2021).
- Köhler, A.; De Filippo, K.; Hasenberg, M.; Van Den Brandt, C.; Nye, E.; Hosking, M.P.; Lane, T.E.; Mann, L.; Ransohoff, R.M.; Hauser, A.E.; et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood 2011, 117, 4349–4357. [Google Scholar] [CrossRef] [Green Version]
- Scholz, M.; Schirm, S.; Wetzler, M.; Engel, C.; Loeffler, M. Pharmacokinetic and -dynamic modelling of G-CSF derivatives in humans. Theor. Biol. Med. Model. 2012, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.J.; Bohlke, K.; Lyman, G.H.; Carson, K.R.; Crawford, J.; Cross, S.J.; Goldberg, J.M.; Khatcheressian, J.L.; Leighl, N.B.; Perkins, C.L.; et al. Recommendations for the use of WBC growth factors: American society of clinical oncology clinical practice guideline update. J. Clin. Oncol. 2015, 33, 3199–3212. [Google Scholar] [CrossRef] [Green Version]
- Seicean, A.; Gheorghiu, M.; Zaharia, T.; Calinici, T.; Samarghitan, A.; Marcus, B.; Cainap, S.; Seicean, R. Performance of the Standard 22G Needle for Endoscopic Ultrasound-guided Tissue Core Biopsy in Pancreatic Cancer. J. Gastrointest Liver Dis. 2016, 25, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Cancer Care Ontario. Cancer Care Ontario GCSF Recommendations 2016; Cancer Care Ontario: Toronto, ON, Canada, 2016. [Google Scholar]
- Fagnani, D.; Isa, L.; Verga, M.F.; Nova, P.; Casartelli, C.; Filipazzi, V.; Danova, M.; Farina, G.; Pugliese, P.; Fava, S.; et al. Granulocyte colony-stimulating factors used in clinical practice: Polonord Registry-Based Cohort Italian Study. Tumori 2014, 100, 491–498. [Google Scholar] [CrossRef]
- Yoshida, M.; Karasawa, M.; Naruse, T.; Fukuda, M.; Hirashima, K.; Oh, H.; Ninomiya, H.; Abe, T.; Saito, K.; Shishido, H.; et al. Effect of granulocyte-colony stimulating factor on empiric therapy with flomoxef sodium and tobramycin in febrile neutropenic patients with hematological malignancies. Int. J. Hematol. 1999, 69, 81–88. Available online: https://pubmed.ncbi.nlm.nih.gov/10071455/ (accessed on 1 April 2021).
- Özkaynak, M.F.; Krailo, M.; Chen, Z.; Feusner, J. Randomized comparison of antibiotics with and without granulocyte colony-stimulating factor in children with chemotherapy-induced febrile neutropenia: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2005, 45, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Soda, H.; Oka, M.; Fukuda, M.; Kinoshita, A.; Sakamoto, A.; Araki, J.; Satoru, F.; Itoh, N.; Watanabe, K.; Kanda, T.; et al. Optimal schedule for administering granulocyte colony-stimulating factor in chemotherapy-induced neutropenia in non-small-cell lung cancer. Cancer Chemother. Pharmacol. 1996, 38, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Soda, H.; Oka, M.; Fukuda, M.; Kinoshita, A.; Sakamoto, A.; Araki, J.; Satoru, F.; Itoh, N.; Watanabe, K.; Kanda, T.; et al. Granulocyte colony-stimulating factor in the treatment of high-risk febrile neutropenia: A multicenter randomized trial. J. Natl. Cancer Inst. 2001, 93, 31–38. [Google Scholar]
- Mhaskar, R.; Clark, O.A.C.; Lyman, G.; Botrel, T.E.A.; Paladini, L.M.; Djulbegovic, B. Colony-stimulating factors for chemotherapy-induced febrile neutropenia (Review) summary of findings for the main comparison. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Neulasta European Medicines Agency. Available online: https://www.ema.europa.eu/en/documents/product-information/neulasta-epar-product-information_en.pdf (accessed on 1 April 2021).
- Nivestim European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/nivestim (accessed on 1 April 2021).
- Shochat, E.; Rom-Kedar, V. Novel strategies for granulocyte colony-stimulating factor treatment of severe prolonged neutropenia suggested by mathematical modeling. Clin. Cancer Res. 2008, 14, 6354–6363. [Google Scholar] [CrossRef] [Green Version]
- Cornes, P.; Gascon, P.; Chan, S.; Hameed, K.; Mitchell, C.R.; Field, P.; Latymer, M.; Arantes, L.H. Systematic Review and Meta-analysis of Short- versus Long-Acting Granulocyte Colony-Stimulating Factors for Reduction of Chemotherapy-Induced Febrile Neutropenia. Adv. Ther. 2018, 35, 1816–1829. Available online: http://link.springer.com/10.1007/s12325-018-0798-6 (accessed on 1 April 2021). [CrossRef] [Green Version]
- Watts, M.J.; Addison, I.; Long, S.G.; Hartley, S.; Warrington, S.; Boyce, M.; Linch, D.C. Crossover study of the haematological effects and pharmacokinetics of glycosylated and non-glycosylated G-CSF in healthy volunteers. Br. J. Haematol. 1997, 98, 474–479. [Google Scholar] [CrossRef]
- Kröger, N.; Renges, H.; Sonnenberg, S.; Krüger, W.; Gutensohn, K.; Dielschneider, T.; Cortes-Dericks, L.; Zander, A.R. Stem cell mobilisation with 16 μg/kg vs 10 μg/kg of G-CSF for allogeneic transplantation in healthy donors. Bone Marrow Transplant. 2002, 29, 727–730. [Google Scholar] [CrossRef] [Green Version]
- Petros, W.P.; Rabinowitz, J.; Stuart, A.; Peters, W.P. Clinical pharmacology of filgrastim following high-dose chemotherapy and autologous bone marrow transplantation. Clin. Cancer Res. 1997, 3, 705–711. [Google Scholar] [PubMed]
- Paul, M.; Ram, R.; Kugler, E.; Farbman, L.; Peck, A.; Leibovici, L.; Lahav, M.; Yeshurun, M.; Shpilberg, O.; Herscovici, C.; et al. Rac signal adaptation controls neutrophil mobilization from the bone marrow. Sci. Signal. 2016, 9, ra124. [Google Scholar]
- Paul, M.; Ram, R.; Kugler, E.; Farbman, L.; Peck, A.; Leibovici, L.; Lahav, M.; Yeshurun, M.; Shpilberg, O.; Herscovici, C.; et al. Subcutaneous versus intravenous granulocyte colony stimulating factor for the treatment of neutropenia in hospitalized hemato-oncological patients: Randomized controlled trial. Am. J. Hematol. 2014, 89, 243–248. [Google Scholar] [CrossRef] [PubMed]

| Item | Number of Patients | N (%) |
|---|---|---|
| Age, mean | 96 | 58.79 |
| Gender | Male | 47 (48.96) |
| Female | 49 (51.04) | |
| BMI, median (IQR) | 73 | 24.05 (21.68–28.1) |
| G-CSF prophylactic before FN episode | 96 | 19/94 (20.21) |
| Type of cancer | colon/rectum | 21 (21.87) |
| gastric | 13 (13.54) | |
| ovarian | 10 (10.42) | |
| lung | 10 (10.42) | |
| head and neck | 11 (11.46) | |
| germinal tumors | 5 (5.20) | |
| other | 26 (27.09) | |
| TNM initial stage of neoplasia | 1 | 5 (7.14) |
| 2 | 15 (21.42) | |
| 3 | 21 (30) | |
| 4 | 29 (41.42) | |
| Chemotherapy with FN episode | line | 2 |
| cycle | 3.6 | |
| Chemotherapy regimen | 96 | |
| platinum-based | 48 (50) | |
| taxane-based | 17 (17.70) | |
| antracycline | 25 (26.04) | |
| other | 6(6.25) | |
| Disease status | controlled | 2 (2.08) |
| evolutive | 94 (97.92) |
| Item | Median (IQR) | 95% CI | Range |
|---|---|---|---|
| aplasia duration in days | 1 (1–3) | 1–2 | 1–8 |
| aplasia duration | 1530 (1449.75–4310.75) | 1490–2861 | 1102–11,513 |
| Disease control | days | ||
| evolutive (N = 94) | 1 (1–3) | p value = 0.238 | |
| partial response (N = 2) | 1 (1–1) | ||
| G-CSF prophylactic administration | days | ||
| yes | 1 (1–3) | p value = 0.598 | |
| no | 1 (1–3) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căinap, C.; Cetean-Gheorghe, S.; Pop, L.A.; Leucuta, D.C.; Piciu, D.; Mester, A.; Vlad, C.; Ovidiu, C.; Gherman, A.; Crişan, C.; et al. Continuous Intravenous Administration of Granulocyte-Colony-Stimulating Factors—A Breakthrough in the Treatment of Cancer Patients with Febrile Neutropenia. Medicina 2021, 57, 675. https://doi.org/10.3390/medicina57070675
Căinap C, Cetean-Gheorghe S, Pop LA, Leucuta DC, Piciu D, Mester A, Vlad C, Ovidiu C, Gherman A, Crişan C, et al. Continuous Intravenous Administration of Granulocyte-Colony-Stimulating Factors—A Breakthrough in the Treatment of Cancer Patients with Febrile Neutropenia. Medicina. 2021; 57(7):675. https://doi.org/10.3390/medicina57070675
Chicago/Turabian StyleCăinap, Călin, Sânziana Cetean-Gheorghe, Laura Ancuta Pop, Daniel Corneliu Leucuta, Doina Piciu, Andra Mester, Cătălin Vlad, Crişan Ovidiu, Alexandra Gherman, Cristina Crişan, and et al. 2021. "Continuous Intravenous Administration of Granulocyte-Colony-Stimulating Factors—A Breakthrough in the Treatment of Cancer Patients with Febrile Neutropenia" Medicina 57, no. 7: 675. https://doi.org/10.3390/medicina57070675
APA StyleCăinap, C., Cetean-Gheorghe, S., Pop, L. A., Leucuta, D. C., Piciu, D., Mester, A., Vlad, C., Ovidiu, C., Gherman, A., Crişan, C., Bereanu, A., Bălăcescu, O., Constantin, A. M., Dicu, I., Bălăcescu, L., Stan, A., Achimaş-Cadariu, P., & Căinap, S. (2021). Continuous Intravenous Administration of Granulocyte-Colony-Stimulating Factors—A Breakthrough in the Treatment of Cancer Patients with Febrile Neutropenia. Medicina, 57(7), 675. https://doi.org/10.3390/medicina57070675








