Why Use Ultrashort Pulses in Ophthalmology and Which Factors Affect Cut Quality
Abstract
:1. Introduction
2. Materials and Methods
3. Ultrashort Pulsed Femtosecond Laser: Mechanism of Laser–Tissue Interaction
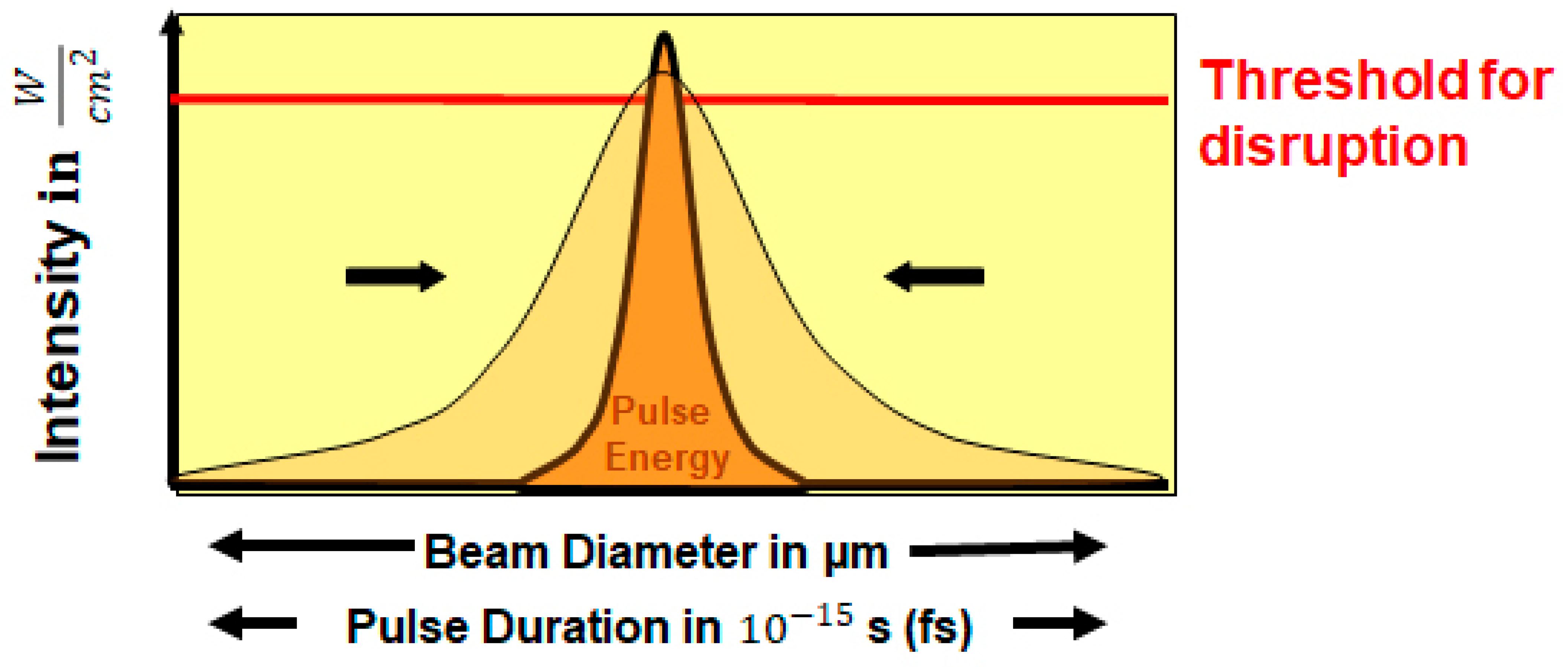
3.1. Photodisruption
3.2. Cutting the Tissue
4. Factors Influencing the Cut Quality
4.1. Pulse Duration
4.2. Laser Beam Size and Beam Focusing
4.3. Femtosecond Laser Wavelength
5. Currently Available Femtosecond Laser Devices
6. Clinical Outcome
7. For the Future—One Laser for All Procedures
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelman, C.D. Phacoemulsification and aspiration. A new technique of cataract removal. A preliminary report. Am. J. Ophthalmol. 1967, 64, 23–35. [Google Scholar] [CrossRef]
- Krasnov, M.M. Laser-phakopuncture in the treatment of soft cataracts. Br. J. Ophthalmol. 1975, 59, 96–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aron-Rosa, D.; Aron, J.J.; Griesemann, M.; Thyzel, R. Use of the neodymium-YAG laser to open the posterior capsule after lens implant surgery: A preliminary report. J. Am. Intraocul. Implant Soc. 1980, 6, 352–354. [Google Scholar] [CrossRef]
- Vogel, A.; Schweiger, P.; Frieser, A.; Asiyo, M.; Birngruber, R. Intraocular Nd: YAG laser surgery: Light-tissue interaction, damage range, and reduction of collateral effects. IEEE J. Quant. Electr. 1990, 26, 2240–2260. [Google Scholar] [CrossRef]
- Zysset, B.; Fujimoto, J.G.; Puliafito, C.A.; Birngruber, R.; Deutsch, T.F. Picosecond optical breakdown: Tissue effects and reduction of collateral damage. Lasers Surg. Med. 1989, 9, 193–204. [Google Scholar] [CrossRef]
- Vogel, A.; Busch, S.; Jungnickel, K.; Birngruber, R. Mechanisms of intraocular photodisruption with picosecond and nanosecond laser pulses. Lasers Surg. Med. 1994, 15, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Docchio, F.; Sacchi, C.A.; Marshall, J. Experimental investigation of optical breakdown thresholds inocular media under single pulse irradiation with different puls durations. Laser Ophthalmol. 1986, 1, 83–93. [Google Scholar]
- Zysset, B.; Fujimoto, G.; Deutsch, T.F. Time resolved measurements of picosecond optical breackdown. Appl. Phys. B 1989, 48, 139–147. [Google Scholar] [CrossRef]
- Vogel, A.; Capon, M.R.; Asiyo-Vogel, M.N.; Birngruber, R. Intraocular photodisruption with picosecond and nanosecond laser pulses: Tissue effects in cornea, lens, and retina. Invest. Ophthalmol. Vis. Sci. 1994, 35, 3032–3044. [Google Scholar]
- Gailitis, R.P.; Patterson, S.W.; Samuels, M.A.; Hagen, K.; Ren, Q.; Waring, G.O., 3rd. Comparison of laser phacovaporization using the Er-YAG and the Er-YSGG laser. Arch. Ophthalmol. 1993, 111, 697–700. [Google Scholar] [CrossRef]
- Fisher, R.F.; Pettet, B.E. Presbyopia and the water content of the human crystalline lens. J. Physiol. 1973, 234, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Q.; Venugopalan, V.; Schomacker, K.; Deutsch, T.F.; Flotte, T.J.; Puliafito, C.A.; Birngruber, R. Mid-infrared laser ablation of the cornea: A comparative study. Lasers Surg. Med. 1992, 12, 274–281. [Google Scholar] [CrossRef]
- Höh, H.; Fischer, E. Pilot study on erbium laser phacoemulsification. Ophthalmology 2000, 107, 1053–1061. [Google Scholar] [CrossRef]
- Höh, H.; Fischer, E. Erbium laser phacoemulsification—A clinical pilot study. Klin. Monbl. Augenheilkd. 1999, 214, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.T., Jr.; Cummings, J.P. Effect of the dynamic optical properties of water on midinfrared laser ablation. Lasers Surg. Med. 1994, 15, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Stern, D.; Puliafito, C.A. High-speed photography of Er: YAG laser ablation in fluid. Implication for laser vitreous surgery. Invest. Ophthalmol. Vis. Sci. 1990, 31, 2546–2550. [Google Scholar]
- Neubaur, C.C.; Stevens, G., Jr. Erbium: YAG laser cataract removal: Role of fiber-optic delivery system. J. Cataract Refract. Surg. 1999, 25, 514–520. [Google Scholar] [CrossRef]
- Dodick, J.M. Laser phacolysis of the human cataractous lens. Dev. Ophthalmol. 1991, 22, 58–64. [Google Scholar]
- Dodick, J.M.; Christiansen, J. Experimental studies on the development and propagation of shock waves created by the interaction of short Nd: YAG laser pulses with a titanium target. Possible implications for Nd: YAG laser phacolysis of the cataractous human lens. J. Cataract Refract. Surg. 1991, 17, 794–797. [Google Scholar] [CrossRef]
- Dodick, J.M.; Sperber, L.T.; Lally, J.M.; Kazlas, M. Neodymium-YAG laser phacolysis of the human cataractous lens. Arch. Ophthalmol. 1993, 111, 903–904. [Google Scholar] [CrossRef]
- Birngruber, R.; Puliafito, C.A.; Gawande, A.; Lin, W.Z.; Schoenlein, R.W.; Fujimoto, J.G. Femtosecond Laser Tissue Interaction—Retina Injury Studies. IEEE J. Quantum Electron. 1987, 23, 1836–1844. [Google Scholar] [CrossRef]
- Kautek, W.; Mitterer, S.; Krueger, J.; Husinsky, W.; Grabner, G. Femtosecond-Pulse Laser Ablation of Human Cornea. Appl. Phys. A 1994, 58, 513–518. [Google Scholar] [CrossRef]
- Ratkay-Traub, I.; Juhasz, T.; Horvath, C.; Suarez, C.; Kiss, K.; Ferencz, I.; Kurtz, R. Ultra-short puls (femtosecond) laser surgery: Initial use in LASIK flap creation. Ophthalmol. Clin. N. Am. 2001, 14, 347–355. [Google Scholar]
- Kim, P.; Sutton, G.L.; Rootman, D.S. Appliacations of femtosecond laser in corneal refractive surgery. Curr. Opin. 2011, 22, 238–244. [Google Scholar]
- Nagy, Z. Intraocular femtosecond laser applications in cataract surgery. Cataract Refracti. Surg. Today 2009, 4, 29–30. [Google Scholar]
- Pajic, B.; Cvejic, Z.; Pajic-Eggspuehler, B. Cataract Surgery Performed by High Frequency LDV Z8 Femtosecond Laser: Safety, Efficacy, and Its Physical Properties. Sensors 2017, 17, 1429. [Google Scholar] [CrossRef] [Green Version]
- Pajic, B.; Vastardis, I.; Pajic-Eggspuehler, B.; Gatzioufas, Z.; Hafezi, F. Femtosecond laser versus mechanical microkeratome-assisted flap creation for LASIK: A prospective, randomized, paired-eye study. Clin. Ophthalmol. 2014, 22, 1883–1889. [Google Scholar]
- Durrie, D.S.; Kerizian, G.M. Femtosecond laser versus mechanical keratome flaps in wavefront-guided laser in situ keratomileusis:Prospective contralateral eye study. J. Cataract Refract. Surg. 2005, 31, 120–126. [Google Scholar] [CrossRef]
- Binder, P.S. One thousand consecutive IntraLase Laser in situ keratomileusis flaps. J. Cataract Refract. Surg. 2006, 32, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Kerizian, G.M.; Stonecipher, K.G. Comparison of IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J. Cataract Refract. Surg. 2004, 30, 804–811. [Google Scholar]
- Tran, D.B.; Sarayba, M.A.; Bor, Z.; Garufis, C.; Duh, Y.J.; Soltes, C.R.; Juhasz, T.; Kurtz, R.M. Randomized prospective clinical study comparing induced aberrations with IntraLase and Hansatome flap creation in fellow eyes: Potential impact on wavefront-guided laser in situ keratomileusis. J. Cataract Refract. Surg. 2005, 31, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Kim, J.K.; Kim, C.K.; Han, G.H.; Seo, K.Y.; Kim, E.K.; Kim, T.I. Comparison of laser in situ keratomileusis flaps created by 3 femtosecond lasers and a microkeratome. J. Cataract Refract. Surg. 2011, 37, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, G.; Albarrán-Diego, C.; Ferrer-Blasco, T.; García-Lázaro, S.; Cerviño-Expósito, A. Long-term comparison of corneal aberration changes after laser in situ keratomileusis: Mechanical microkeratome versus femtosecond laser flap creation. J. Cataract Refract. Surg. 2010, 36, 1934–1944. [Google Scholar] [CrossRef]
- Moshirfar, M.; Gardiner, J.P.; Schliesser, J.A.; Espandar, L.; Feiz, V.; Mifflin, M.D.; Chang, J.C. Laser in situ keratomileusis flap complications using mechanical microkeratome versus femtosecond laser: Retrospective comparison. J. Cataract Refract. Surg. 2010, 36, 1925–1933. [Google Scholar] [CrossRef]
- Conrad-Hengerer, I.; Hengerer, F.H.; Schultz, T.; Dick, H.B. Effect of femtosecond laser fragmentation on effective phacoemulsification time in cataract surgery. J. Refract. Surg. 2012, 28, 879–883. [Google Scholar] [CrossRef]
- Friedman, N.J.; Palanker, D.V.; Schuele, G.; Andersen, D.; Marcellino, G.; Seibel, B.S.; Batlle, J.; Feliz, R.; Talamo, J.H.; Blumenkranz, M.S.; et al. Femtosecond laser capsulotomy. J. Cataract Refract. Surg. 2011, 37, 1189–1198. [Google Scholar] [CrossRef]
- Abell, R.G.; Kerr, N.M.; Vote, B.J. Toward zero effective phacoemulsification time using femtosecond laser pretreatment. Ophthalmology 2013, 120, 942–948. [Google Scholar] [CrossRef]
- Grewal, D.S.; Basti, S. Comparison of morphologic features of clear corneal incisions created with a femtosecond laser or a keratome. J. Cataract Refract. Surg. 2014, 40, 521–530. [Google Scholar] [CrossRef]
- Alió, J.L.; Abdou, A.A.; Soria, F.; Javaloy, J.; Fernández-Buenaga, R.; Nagy, Z.Z.; Filkorn, T. Femtosecond laser cataract incision morphology and corneal higher-order aberration analysis. J. Refract. Surg. 2013, 29, 590–595. [Google Scholar] [CrossRef]
- Mastropasqua, L.; Toto, L.; Mastropasqua, A.; Vecchiarino, L.; Mastropasqua, R.; Pedrotti, E.; Di Nicola, M. Femtosecond laser versus manual clear corneal incision in cataract surgery. J. Refract. Surg. 2014, 30, 27–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.V.; Lawless, M.; Bali, S.J.; Hodge, C.; Sutton, G. Surgical outcomes and safety of femtosecond laser cataract surgery: A prospective study of 1500 consecutive cases. Ophthalmology 2013, 120, 227–233. [Google Scholar] [CrossRef]
- Nagy, Z.Z.; Takacs, A.I.; Filkorn, T.; Kránitz, K.; Gyenes, A.; Juhász, É.; Sándor, G.L.; Kovacs, I.; Juhász, T.; Slade, S. Complications of femtosecond laser-assisted cataract surgery. J. Cataract Refract. Surg. 2014, 40, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Noack, J.; Nahen, K.; Theisen, D.; Busch, S.; Parlitz, U.; Hammer, D.X.; Noojin, G.D.; Rockwell, B.A.; Birngruber, R. Energy balance of optical breakdown in water at nanosecond to femtosecond time scales. Appl. Phys. B 1999, 68, 271–280. [Google Scholar] [CrossRef]
- Niemz, M.H. Laser-Tissue Interactions: Fundamentals and Applications, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Vogel, A.; Noack, J.; Huettmann, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Riau, A.K.; Liu, Y.C.; Lwin, N.C.; Ang, H.P.; Tan, N.Y.S.; Yam, G.H.F.; Tan, D.T.; Mehta, J.S. Comparative study of nJ- and μJ-energy level femtosecond lasers: Evaluation of flap adhesion strength, stromal bed quality, and tissue responses. Invest. Ophthalmol. Vis. Sci. 2014, 55, 3186–3194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A. Nonlinear absorption: Intraocular microsurgery and laser lithotripsy. Phys. Med. Biol 1997, 42, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.; Riemann, I.; Fritzsche, W. Nanodissection of human chromosomeswith near-infrared femtosecond laser Pulses. Opt. Lett. 2001, 26, 819–821. [Google Scholar] [CrossRef]
- Lubatschowski, H. Update on fs laser technology in ophthalmology. Klin. Monbl. Augenheilkd. 2013, 230, 1207–1212. [Google Scholar]
- Krueger, R.R.; Talamo, J.H.; Lindstrom, R.L. Textbook of refractive Laser assisted Cataract surgery (ReLACS). In Chapter 3: Femtosecond Laser Fundamentals; Springer: New York, NY, USA; Heidelberg, Germany, 2012. [Google Scholar]
- Pajic, B.; Vastardis, I.; Gatzioufas, Z.; Pajic-Eggspuehler, B. First experience with the new high-frequency femtosecond laser system (LDV Z8) for cataract surgery. Clin. Ophthalmol. 2014, 8, 2485–2489. [Google Scholar] [CrossRef] [Green Version]
- Crotti, C.; Deloison, F.; Alahyane, F.; Aptel, F.; Kowalczuk, L.; Legeais, J.M.; Peyrot, D.A.; Savoldelli, M.; Plamann, K. Wavelength optimization in femtosecond laser corneal surgery. Invest. Ophthalmol. Vis. Sci. 2013, 54, 3340–3349. [Google Scholar] [CrossRef]
- Hammer, C.M.; Petsch, C.; Klenke, J.; Skerl, K.; Paulsen, F.; Kruse, F.E.; Seiler, T.; Menzel-Severing, J. Corneal tissue interactions of a new 345 nm ultraviolet femtosecond laser. J. Cataract Refract. Surg. 2015, 41, 1279–1288. [Google Scholar] [CrossRef] [Green Version]
- Vogel, A.; Linz, N.; Freidank, S.; Paltauf, G. Femtosecond-laser-induced nanocavitation in water:implications for optical breakdown threshold and cell surgery. Phys. Rev. Lett. 2008, 100, 038102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasavada, A.; Singh, R.; Desai, J. Phacoemulsification of white mature cataracts. J. Cataract Refract. Surg. 1998, 24, 270–277. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Singh, S. Phacoemulsification in eyes with white cataract. J. Cataract Refract. Surg. 2000, 26, 1041–1047. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Natkunarajah, M.; Riaz, Y.; Bourne, R.R.; Restori, M.; Allan, B.D. Biometry and formula accuracy with intraocular lenses used for cataract surgery in extreme hyperopia. Am. J. Ophthalmol. 2007, 143, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.F.; Marques, D.M.; Osher, R.H.; Osher, J.M. Fate of anterior capsule tears during cataract surgery. J. Cataract Refract. Surg. 2006, 32, 1638–1642. [Google Scholar] [CrossRef]
- Lundström, M.; Behndig, A.; Montan, P.; Artzén, D.; Jakobsson, G.; Johansson, B.; Thorburn, W.; Stenevi, U. Capsule complication during cataract surgery: Background, study design, and required additional care: Swedish Capsule Rupture Study Group report 1. J. Cataract Refract. Surg. 2009, 35, 1679–1687. [Google Scholar] [CrossRef]
- Conrad-Hengerer, I.; Hengerer, F.H.; Joachim, S.C.; Schultz, T.; Dick, H.B. Femtosecond laser-assisted cataract surgery in intumescent white cataracts. J. Cataract Refract. Surg. 2014, 40, 44–50. [Google Scholar] [CrossRef]
- Schultz, T.; Dick, H.B. Laser-assisted mini-capsulotomy: A new technique for intumescent white cataracts. J. Refract. Surg. 2014, 30, 742–745. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.Z.; Kránitz, K.; Takacs, A.; Filkorn, T.; Gergely, R.; Knorz, M.C. Intraocular femtosecond laser use in traumatic cataracts following penetrating and blunt trauma. J. Refract. Surg. 2012, 28, 151–153. [Google Scholar] [CrossRef] [Green Version]
- Schultz, T.; Ezeanosike, E.; Dick, H.B. Femtosecond laser-assisted cataract surgery in pediatric Marfan syndrome. J. Refract. Surg. 2013, 29, 650–652. [Google Scholar] [CrossRef]
- Chang, D.F.; Campbell, J.R. Intraoperative floppy iris syndrome associated with tamsulosin. J. Cataract Refract. Surg. 2005, 31, 664–673. [Google Scholar] [CrossRef]
- Chang, D.F.; Osher, R.H.; Wang, L.; Koch, D.D. Prospective multicenter evaluation of cataract surgery in patients taking tamsulosin (Flomax). Ophthalmology 2007, 114, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.F.; Braga-Mele, R.; Mamalis, N.; Masket, S.; Miller, K.M.; Nichamin, L.D.; Packard, R.B.; Packer, M. Clinical experience with intraoperative floppy-iris syndrome. Results of the 2008 ASCRS member survey. J. Cataract Refract. Surg. 2008, 34, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D.V.; Blumenkranz, M.S.; Andersen, D.; Wiltberger, M.; Marcellino, G.; Gooding, P.; Angeley, D.; Schuele, G.; Woodley, B.; Simoneau, M.; et al. Femtosecond laser-assisted cataract surgery with integrated optical coherence tomography. Sci. Transl. Med. 2010, 2, 58ra85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kránitz, K.; Takacs, A.; Miháltz, K.; Kovács, I.; Knorz, M.C.; Nagy, Z.Z. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J. Refract. Surg. 2011, 27, 558–563. [Google Scholar] [CrossRef] [Green Version]
- Auffarth, G.U.; Reddy, K.P.; Ritter, R.; Holzer, M.P.; Rabsilber, T.M. Comparison of the maximum applicable stretch force after femtosecond laser-assisted and manual anterior capsulotomy. J. Cataract Refract. Surg. 2013, 39, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, A.; Kropp, M.; Cvejic, Z.; Thumann, G.; Pajic, B. Comparison of femtosecond laser-assisted and ultrasound-assisted cataract surgery with focus on endothelial analysis. Sensors 2021, 21, 996. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pajic, B.; Pajic-Eggspuehler, B.; Rathjen, C.; Resan, M.; Cvejic, Z. Why Use Ultrashort Pulses in Ophthalmology and Which Factors Affect Cut Quality. Medicina 2021, 57, 700. https://doi.org/10.3390/medicina57070700
Pajic B, Pajic-Eggspuehler B, Rathjen C, Resan M, Cvejic Z. Why Use Ultrashort Pulses in Ophthalmology and Which Factors Affect Cut Quality. Medicina. 2021; 57(7):700. https://doi.org/10.3390/medicina57070700
Chicago/Turabian StylePajic, Bojan, Brigitte Pajic-Eggspuehler, Christian Rathjen, Mirko Resan, and Zeljka Cvejic. 2021. "Why Use Ultrashort Pulses in Ophthalmology and Which Factors Affect Cut Quality" Medicina 57, no. 7: 700. https://doi.org/10.3390/medicina57070700
APA StylePajic, B., Pajic-Eggspuehler, B., Rathjen, C., Resan, M., & Cvejic, Z. (2021). Why Use Ultrashort Pulses in Ophthalmology and Which Factors Affect Cut Quality. Medicina, 57(7), 700. https://doi.org/10.3390/medicina57070700







