Chorioretinal Folds in the Trabeculectomized Eye with Normal Intraocular Pressure after Phacoemulsification
Abstract
:1. Introduction
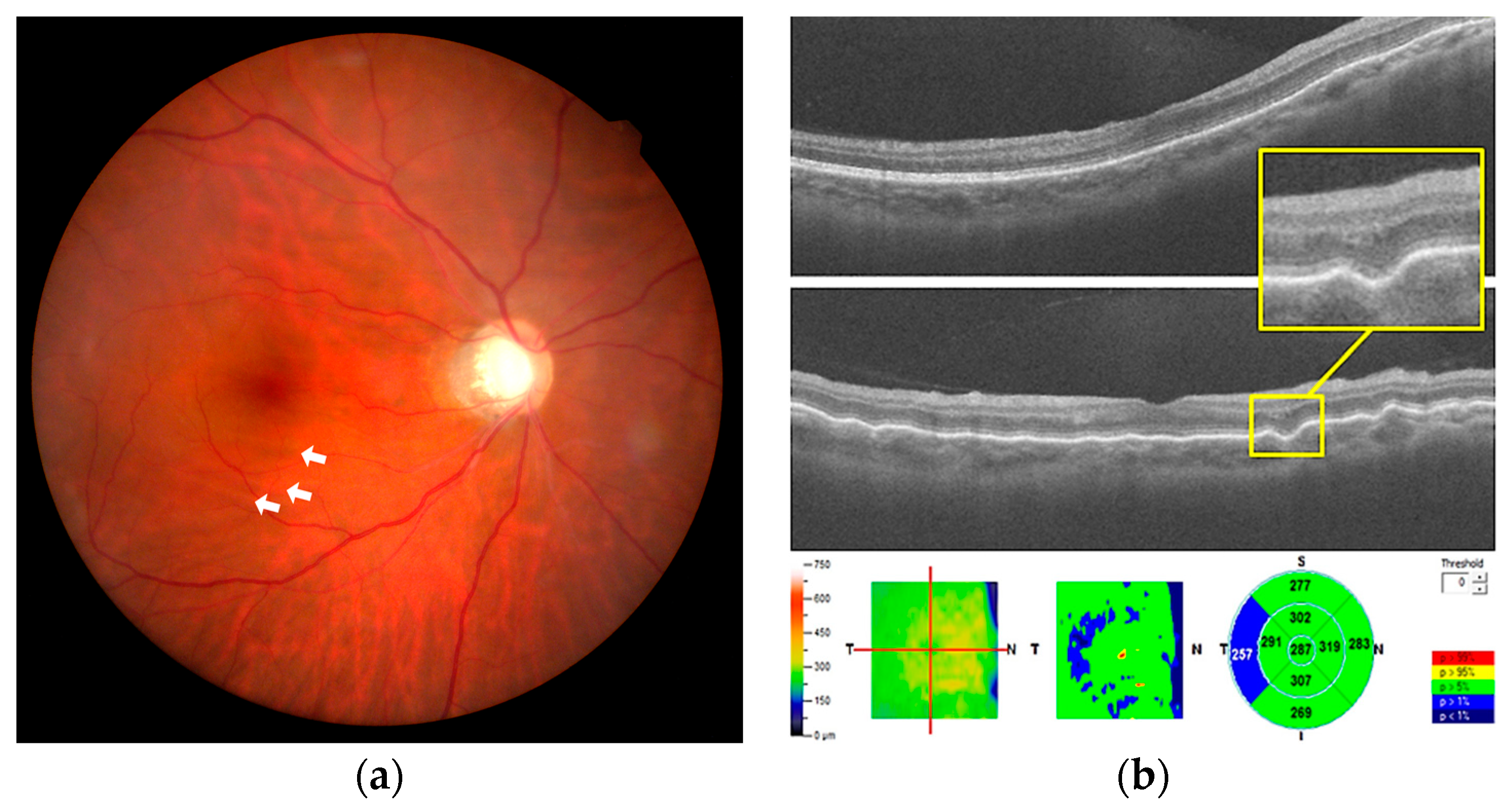
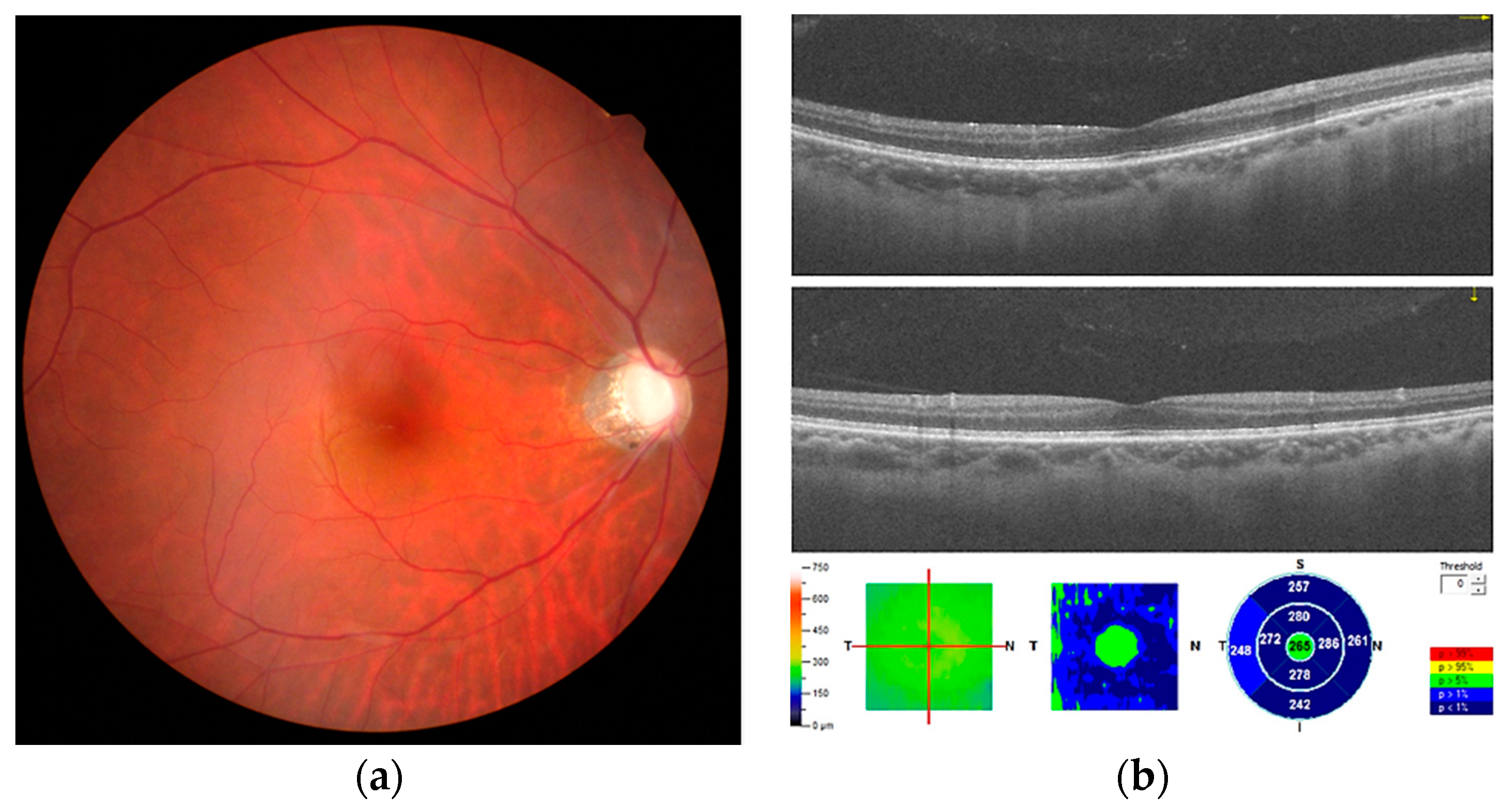
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giocanti-Auregan, A.; Lavia, C.; Gaudric, A.; Grenet, T.; Cohen, S.Y. Staphyloma-related chorioretinal folds. Am. J. Ophthalmol. Case Rep. 2020, 19, 100747. [Google Scholar] [CrossRef]
- Roelofs, K.A.; Eliott, D.; Freitag, S.K. Central Retinal Vein Occlusion with Chorioretinal Folds Secondary to Active Thyroid Eye Disease. Ophthalmology 2018, 125, 1645. [Google Scholar] [CrossRef] [Green Version]
- Vahdani, K.; Rose, G.E. Chorioretinal Folds in Thyroid Eye Disease. Ophthalmology 2019, 126, 1106. [Google Scholar] [CrossRef]
- Cohen, S.Y.; Ducos de Lahitte, G.; Gaudric, A.; Mrejen, S. Chorioretinal folds in patients with central serous chorioretinopathy. Retin. Cases Brief Rep. 2019. [Google Scholar] [CrossRef]
- Kurokawa, T.; Hamano, H.; Muraki, T.; Uehara, T.; Masuo, S.; Murata, T. Immunoglobulin G4-related dacyroadenitis presenting as bilateral chorioretinal folds from severely enlarged lacrimal glands. Am. J. Ophthalmol. Case Rep. 2018, 9, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Nichani, P.; Micieli, J.A. Retinal Manifestations of Idiopathic Intracranial Hypertension. Ophthalmol. Retina 2021, 5, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Grinager, H.S.; Krason, D.A.; Olsen, T.W. Lyme disease: Resolution of a serous retinal detachment and chorioretinal folds after antibiotic therapy. Retin. Cases Brief Rep. 2012, 6, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Leahey, A.B.; Brucker, A.J.; Wyszynski, R.E.; Shaman, P. Chorioretinal folds. A comparison of unilateral and bilateral cases. Arch. Ophthalmol. 1993, 111, 357–359. [Google Scholar] [CrossRef]
- Cagini, C.; Fiore, T.; Iaccheri, B.; Piccinelli, F.; Ricci, M.A.; Fruttini, D. Macular thickness measured by optical coherence tomography in a healthy population before and after uncomplicated cataract phacoemulsification surgery. Curr. Eye Res. 2009, 34, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Gharbiya, M.; Cruciani, F.; Cuozzo, G.; Parisi, F.; Russo, P.; Abdolrahimzadeh, S. Macular thickness changes evaluated with spectral domain optical coherence tomography after uncomplicated phacoemulsification. Eye 2013, 27, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Šiško, K.; Knez, N.K.; Pahor, D. Influence of cataract surgery on macular thickness: A 6-month follow-up. Wien. Klin. Wochenschr. 2015, 127 (Suppl. 5), S169–S174. [Google Scholar] [CrossRef]
- Hwang, H.S.; Ahn, Y.J.; Lee, H.J.; Kim, M.S.; Kim, E.C. Comparison of macular thickness and inflammatory cytokine levels after microincision versus small incision coaxial cataract surgery. Acta Ophthalmol. 2016, 94, e189–e194. [Google Scholar] [CrossRef] [Green Version]
- Friberg, T.R. The etiology of choroidal folds. A biomechanical explanation. Graefes Arch. Clin. Exp. Ophthalmol. 1989, 227, 459–464. [Google Scholar] [CrossRef]
- Sato, T.; Kohmoto, R.; Fukumoto, M.; Morishita, S.; Kimura, D.; Tajiri, K.; Kobayashi, T.; Kida, T.; Kojima, S.; Ikeda, T. A Case of Diabetic Macular Edema with Prominent Chorioretinal Folds. Case Rep. Ophthalmol. 2017, 8, 163–169. [Google Scholar] [CrossRef]
- Norton, T.T.; Rada, J.A. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995, 35, 1271–1281. [Google Scholar] [CrossRef]
- McBrien, N.A.; Cornell, L.M.; Gentle, A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2179–2187. [Google Scholar]
- Sergienko, N.M.; Shargorogska, I. The scleral rigidity of eyes with different refractions. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1009–1012. [Google Scholar] [CrossRef]
- Roberts, C.J.; Reinstein, D.Z.; Archer, T.J.; Mahmoud, A.M.; Gobbe, M.; Lee, L. Comparison of ocular biomechanical response parameters in myopic and hyperopic eyes using dynamic bidirectional applanation analysis. J. Cataract Refract. Surg. 2014, 40, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.T.; Lee, S.H.; Chen, Y.C. Late-onset Hypotony Maculopathy After Trabeculectomy in a Highly Myopic Patient With Juvenile Open-angle Glaucoma. J. Glaucoma 2017, 26, e137–e141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagnis, A.; Cutolo, C.A.; Corallo, G.; Musetti, D.; Nicolò, M.; Traverso, C.E. Chorioretinal folds: A proposed diagnostic algorithm. Int. Ophthalmol. 2019, 39, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Rada, J.A.; Shelton, S.; Norton, T.T. The sclera and myopia. Exp. Eye Res. 2006, 82, 185–200. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-F.; Chen, C.-L.; Huang, K.-H.; Chen, Y.-H. Chorioretinal Folds in the Trabeculectomized Eye with Normal Intraocular Pressure after Phacoemulsification. Medicina 2021, 57, 896. https://doi.org/10.3390/medicina57090896
Lai Y-F, Chen C-L, Huang K-H, Chen Y-H. Chorioretinal Folds in the Trabeculectomized Eye with Normal Intraocular Pressure after Phacoemulsification. Medicina. 2021; 57(9):896. https://doi.org/10.3390/medicina57090896
Chicago/Turabian StyleLai, Yi-Fen, Ching-Long Chen, Ke-Hao Huang, and Yi-Hao Chen. 2021. "Chorioretinal Folds in the Trabeculectomized Eye with Normal Intraocular Pressure after Phacoemulsification" Medicina 57, no. 9: 896. https://doi.org/10.3390/medicina57090896





