Left Ventricular Systolic Function Has Strong Independent Genetic Background from Diastolic Function: A Classical Twin Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography Measurements
2.3. Statistics
3. Results
3.1. Patient Demographics
3.2. Echocardiography Results
3.3. Genetic Structural Equations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial strain imaging: How useful is it in clinical decision making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanton, T.; Leano, R.; Marwick, T.H. Prediction of all-cause mortality from global longitudinal speckle strain: Comparison with ejection fraction and wall motion scoring. Circ. Cardiovasc. Imaging 2009, 2, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Molnár, A.Á.; Kolossváry, M.; Szilveszter, B.; Panajotu, A.; Lakatos, B.K.; Littvay, L.; Tárnoki, Á.D.; Tárnoki, D.L.; Voros, S.; et al. Genetically determined pattern of left ventricular function in normal and hypertensive hearts. J. Clin. Hypertens. 2018, 20, 949–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; McCabe, E.L.; Larson, M.G.; Chen, M.H.; Osypiuk, E.; Lehman, B.T.; Stantchev, P.; Araga, J.; Solomon, S.D.; Benjamin, E.J.; et al. Left ventricular mechanical function: Clinical correlates, heritability, and association with parental heart failure. Eur. J. Heart Fail. 2015, 17, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, E.R.; Klos, K.L.; Penman, A.D.; Blair, G.J.; Blossom, B.D.; Arnett, D.; Devereux, R.B.; Samdarshi, T.; Boerwinkle, E.; Mosley, T.H., Jr. Heritability and genetic linkage of left ventricular mass, systolic and diastolic function in hypertensive African Americans (from the GENOA Study). Am. J. Hypertens. 2010, 23, 870–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.S.; Kim, K.Y.A.; Peng, J.; Aguilar, F.G.; Selvaraj, S.; Martinez, E.E.; Baldridge, A.S.; Sha, J.; Irvin, M.R.; Broeckel, U.; et al. Clinical correlates and heritability of cardiac mechanics: The HyperGEN study. Int. J. Cardiol. 2019, 274, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Tarnoki, D.L.; Tarnoki, A.D.; Horváth, T.; Jermendy, A.L.; Kolossváry, M.; Szilveszter, B.; Voros, V.; Kovács, A.; Molnár, A.Á.; et al. Rationale, design, and methodological aspects of the BUDAPEST-GLOBAL Study (burden of atherosclerotic plaques study in twins-genetic loci and the burden of atherosclerotic lesions). Clin. Cardiol. 2015, 38, 699–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Littvay, L.; Métneki, J.; Tárnoki, A.D.; Tárnoki, D.L. The Hungarian Twin Registry. Twin Res. Hum. Genet. 2013, 16, 185–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar] [PubMed]
- Franic, S.; Dolan, C.V.; Borsboom, D.; Boomsma, D.I. Structural Equation Modeling in Genetics; Guilford Press: New York, NY, USA, 2012; pp. 617–635. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Neale, M.C.; Hunter, M.D.; Pritikin, J.N.; Zahery, M.; Brick, T.R.; Kirkpatrick, R.M.; Estabrook, R.; Bates, T.C.; Maes, H.H.; Boker, S.M. OpenMx 2.0: Extended Structural Equation and Statistical Modeling. Psychometrika 2016, 81, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, Y.; Oishi, Y.; Miyoshi, H.; Iuchi, A.; Nagase, N.; Oki, T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: A study with two-dimensional strain imaging. J. Am. Soc. Echocardiogr. 2008, 21, 1138–1144. [Google Scholar] [PubMed]
- Wells, Q.S.; Veatch, O.J.; Fessel, J.P.; Joon, A.Y.; Levinson, R.T.; Mosley, J.D.; Held, E.P.; Lindsay, C.S.; Shaffer, C.M.; Weeke, P.E.; et al. Genome-wide association and pathway analysis of left ventricular function after anthracycline exposure in adults. Pharm. Genom. 2017, 27, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.A.; Fetterman, K.A.; Burridge, P.W. HiPSCs in cardio-oncology: Deciphering the genomics. Cardiovasc. Res. 2019, 115, 935–948. [Google Scholar] [CrossRef] [PubMed]
- DeVore, A.D.; McNulty, S.; Alenezi, F.; Ersboll, M.; Vader, J.M.; Oh, J.K.; Lin, G.; Redfield, M.M.; Lewis, G.; Semigran, M.J.; et al. Impaired Left Ventricular Global Longitudinal Strain in Patients with Heart Failure with Preserved Ejection Fraction: Insights from the RELAX Trial. Eur. J. Heart Fail. 2017, 19, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Kuznetsova, T.; Bochud, M.; Richart, T.; Thijs, L.; Cusi, D.; Fagard, R.; Staessen, J.A. Heritability of left ventricular structure and function in Caucasian families. Eur. J. Echocardiogr. 2011, 12, 326–332. [Google Scholar] [PubMed]
- Noh, H.M.; Lee, S.C.; Park, S.W.; Sung, J.; Song, Y.M. Genetic Influence on Left Ventricular Structure and Function: A Korean Twin and Family Study. Twin Res. Hum. Genet. 2015, 18, 281–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasan, R.S.; Glazer, N.L.; Felix, J.F.; Lieb, W.; Wild, P.S.; Felix, S.B.; Watzinger, N.; Larson, M.G.; Smith, N.L.; Dehghan, A.; et al. Genetic variants associated with cardiac structure and function: A meta-analysis and replication of genome-wide association data. JAMA 2009, 302, 168–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, A.C.; Nyholt, D.R.; Neuman, R.; Madden, P.A.F.; Bucholz, K.K.; Todd, R.D.; Nelson, E.C.; Montgomery, G.W.; Martin, N.G. Zygosity diagnosis in the absence of genotypic data: An approach using latent class analysis. Twin Res. 2003, 6, 22–26. [Google Scholar] [CrossRef] [PubMed]
| Variable | GLS | GCS | Basal Rotation | Apical Rotation |
|---|---|---|---|---|
| Common genetic and environmental factors | ||||
| Genetic factors (AC) | 0% | 0% | 0% | 0% |
| Environmental factors (EC) | 0% | 0% | 0% | 0% |
| Specific genetic and environmental factors | ||||
| Genetic factors (AS) | 76% | 77% | 57% | 74% |
| Environmental factors (ES) | 24% | 23% | 43% | 36% |
| Overall contribution of genetic and environmental factors | ||||
| Genetic factors (A) | 76% | 77% | 57% | 74% |
| Environmental factors (E) | 24% | 23% | 43% | 36% |
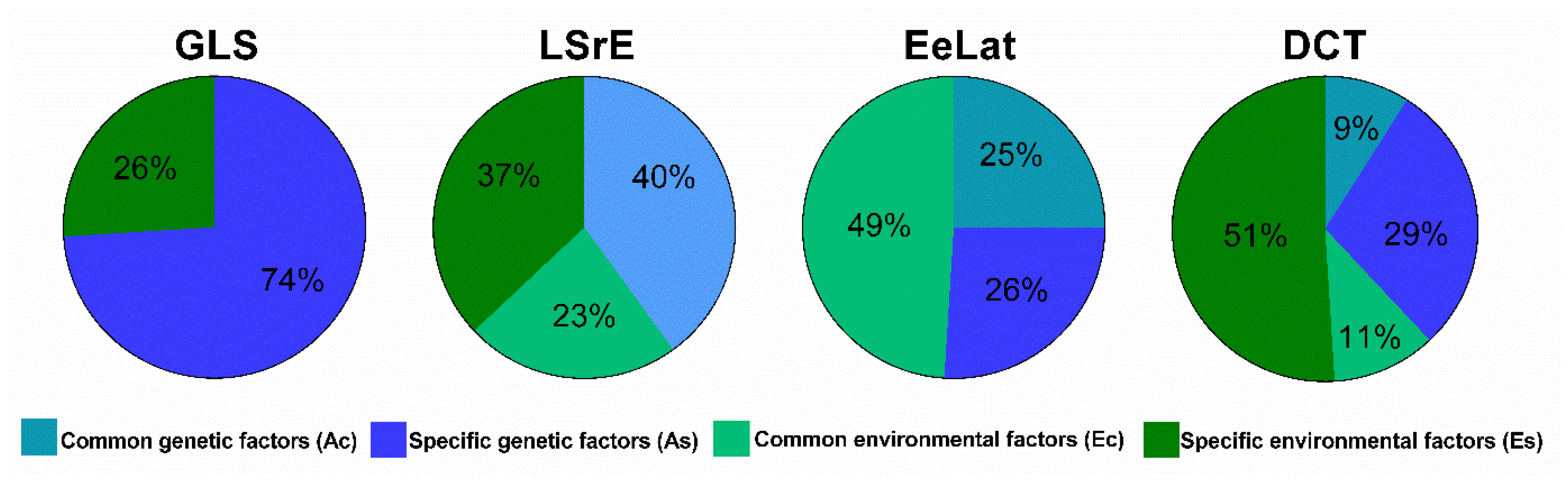
| Variable | LSrE | EeLat | DCT |
|---|---|---|---|
| Common genetic and environmental factors | |||
| Genetic factors (AC) | 40% | 25% | 9% |
| Environmental factors (EC) | 23% | 49% | 11% |
| Specific genetic and environmental factors | |||
| Genetic factors (AS) | 0% | 26% | 29% |
| Environmental factors (ES) | 37% | 0% | 51% |
| Overall contribution of genetic and environmental factors | |||
| Genetic factors (A) | 40% | 51% | 38% |
| Environmental factors (E) | 60% | 49% | 62% |
| Variable | GLS | LSrE | EeLat | DCT |
|---|---|---|---|---|
| Common genetic and environmental factors | ||||
| Genetic factors (AC) | 0% | 40% | 25% | 9% |
| Environmental factors (EC) | 0% | 23% | 49% | 11% |
| Specific genetic and environmental factors | ||||
| Genetic factors (AS) | 74% | 0% | 26% | 29% |
| Environmental factors (ES) | 26% | 37% | 0% | 51% |
| Overall contribution of genetic and environmental factors | ||||
| Genetic factors (A) | 74% | 40% | 51% | 38% |
| Environmental factors (E) | 26% | 60% | 49% | 62% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnár, A.Á.; Kolossváry, M.; Lakatos, B.; Tokodi, M.; Tárnoki, Á.D.; Tárnoki, D.L.; Kovács, A.; Szilveszter, B.; Voros, S.; Jermendy, G.; et al. Left Ventricular Systolic Function Has Strong Independent Genetic Background from Diastolic Function: A Classical Twin Study. Medicina 2021, 57, 935. https://doi.org/10.3390/medicina57090935
Molnár AÁ, Kolossváry M, Lakatos B, Tokodi M, Tárnoki ÁD, Tárnoki DL, Kovács A, Szilveszter B, Voros S, Jermendy G, et al. Left Ventricular Systolic Function Has Strong Independent Genetic Background from Diastolic Function: A Classical Twin Study. Medicina. 2021; 57(9):935. https://doi.org/10.3390/medicina57090935
Chicago/Turabian StyleMolnár, Andrea Ágnes, Márton Kolossváry, Bálint Lakatos, Márton Tokodi, Ádám Domonkos Tárnoki, Dávid László Tárnoki, Attila Kovács, Bálint Szilveszter, Szilard Voros, György Jermendy, and et al. 2021. "Left Ventricular Systolic Function Has Strong Independent Genetic Background from Diastolic Function: A Classical Twin Study" Medicina 57, no. 9: 935. https://doi.org/10.3390/medicina57090935
APA StyleMolnár, A. Á., Kolossváry, M., Lakatos, B., Tokodi, M., Tárnoki, Á. D., Tárnoki, D. L., Kovács, A., Szilveszter, B., Voros, S., Jermendy, G., Maurovich-Horvat, P., & Merkely, B. (2021). Left Ventricular Systolic Function Has Strong Independent Genetic Background from Diastolic Function: A Classical Twin Study. Medicina, 57(9), 935. https://doi.org/10.3390/medicina57090935








