Contrast-Enhanced Ultrasound Qualitative and Quantitative Characteristics of Parathyroid Gland Lesions
Abstract
:1. Introduction
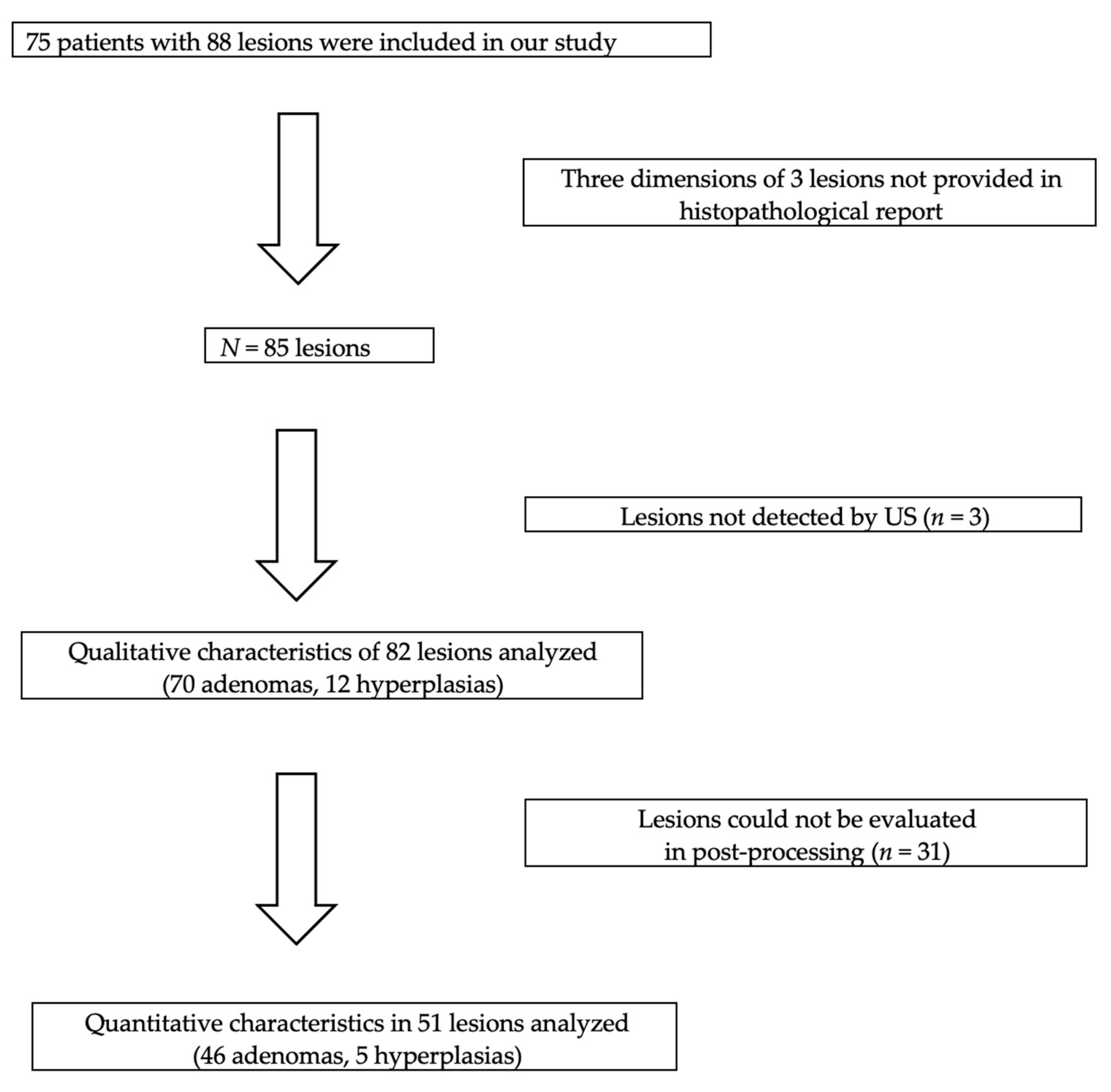
2. Materials and Methods
2.1. Study Design and Population
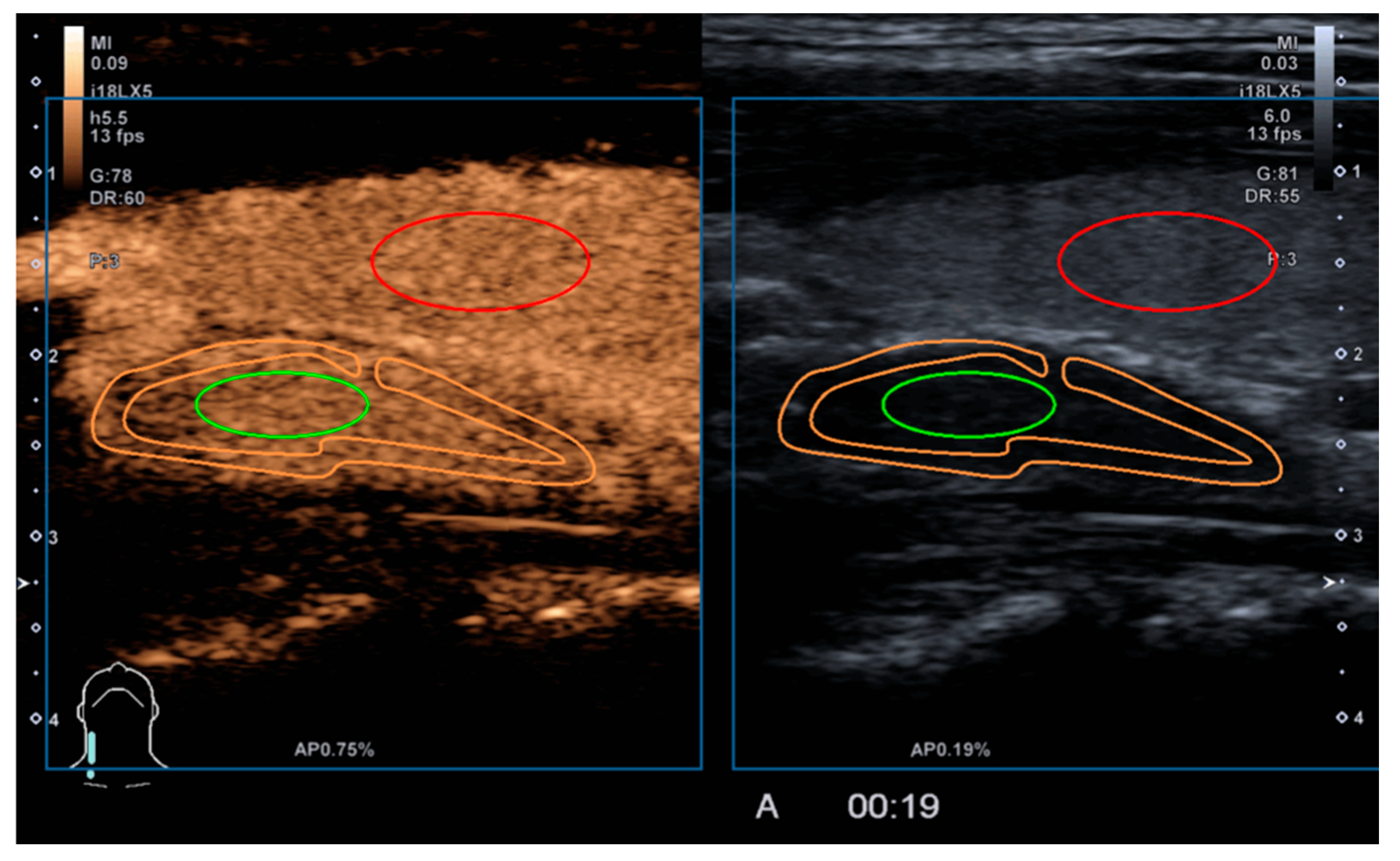
2.2. Study Methodology
2.3. Qualitative Parameters
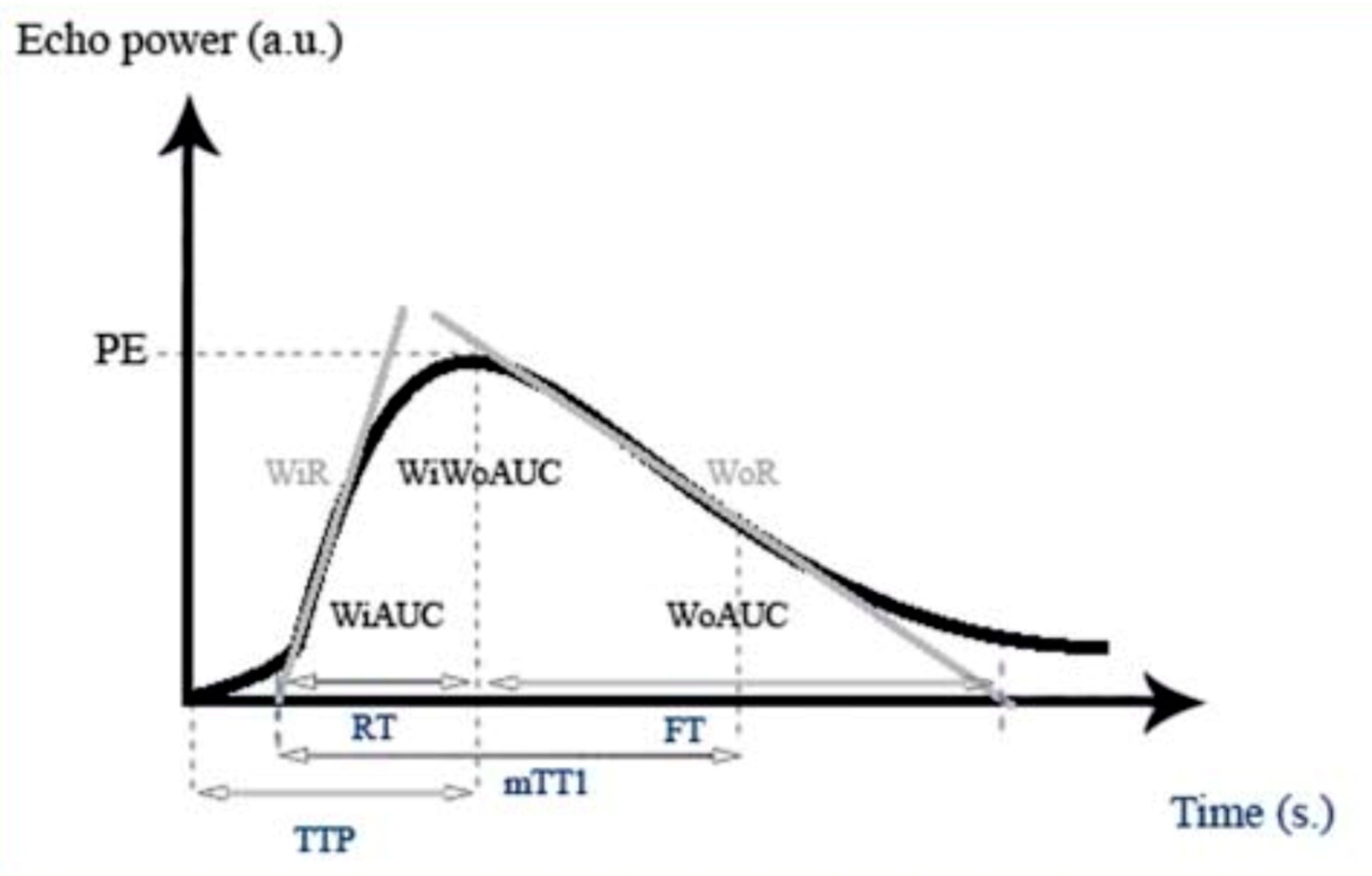
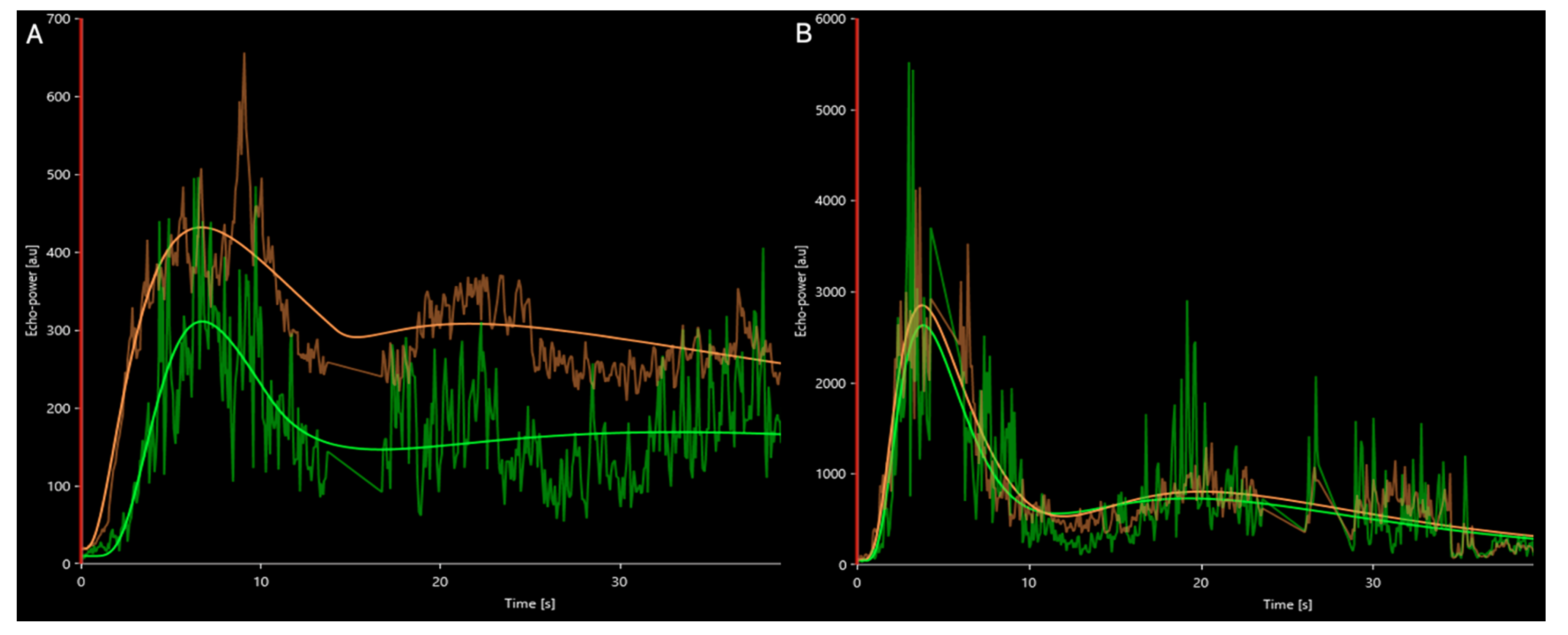
2.4. Quantitative Parameters
2.5. Surgery
2.6. Statistical Analysis
3. Results
3.1. Qualitative Parameters
3.2. Quantitative Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scerrino, G.; Attard, M.; Piccolo, C.L.; Attard, A.; Melfa, G.; Raspanti, C.; Zarcone, M.; Bonventre, S.; Mazzola, S.; Gulotta, G. The coexistence of primary hyperparathyroidism and thyroid nodules: Should the preoperative work-up of the parathyroid and the thyroid diseases be specifically adjusted? Il G. Di Chir. 2016, 37, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Walker, M.; Silverberg, S.J. Primary hyperparathyroidism. Nat. Rev. Endocrinol. 2018, 14, 115–125. [Google Scholar] [CrossRef]
- Guilmette, J.; Sadow, P.M. Parathyroid Pathology. Surg. Pathol. Clin. 2019, 12, 1007–1019. [Google Scholar] [CrossRef]
- Chandran, M.; Wong, J. Secondary and tertiary hyperparathyroidism in chronic kidney disease: An endocrine and renal perspective. Indian J. Endocrinol. Metab. 2019, 23, 391–399. [Google Scholar] [CrossRef]
- Insogna, K.L. Primary Hyperparathyroidism. N. Engl. J. Med. 2018, 379, 1050–1059. [Google Scholar] [CrossRef]
- Uller, W.; Jung, E.; Hornung, M.; Ross, C.; Jung, W.; Schlitt, H.; Stroszczynski, C.; Agha, A. Evaluation of the microvascularization of pathologic parathyroid glands in patients with primary hyperparathyroidism using conventional ultrasound and contrast-enhanced ultrasound. Clin. Hemorheol. Microcirc. 2011, 48, 95–103. [Google Scholar] [CrossRef]
- Sung, J.Y. Parathyroid ultrasonography: The evolving role of the radiologist. Ultrasonography 2015, 34, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Polat, A.V.; Ozturk, M.; Akyuz, B.; Celenk, C.; Kefeli, M.; Polat, C. The diagnostic value of shear wave elastography for parathyroid lesions and comparison with cervical lymph nodes. Med. Ultrason. 2017, 19, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liao, Q.; Wang, Y.; Hu, Y.; Zhu, Q.; Wang, L.; Liu, Q.; Li, J.; Jiang, Y. A new tool for diagnosing parathyroid lesions: Angio plus ultrasound imaging. J. Thorac. Dis. 2019, 11, 4829–4834. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Moloudi, F.; Ghasemi-Rad, M. The role of colour Doppler ultrasonography in the preoperative localization of parathyroid adenomas. Endocr. J. 2012, 59, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azizi, G.; Piper, K.; Keller, J.M.; Mayo, M.L.; Puett, D.; Earp, K.M.; Malchoff, C.D. Shear wave elastography and parathyroid adenoma: A new tool for diagnosing parathyroid adenomas. Eur. J. Radiol. 2016, 85, 1586–1593. [Google Scholar] [CrossRef] [Green Version]
- Yen, H.-H. Progress in the ultrasonographic microvascular imaging. J. Med. Ultrasound 2018, 26, 1–2. [Google Scholar] [CrossRef]
- Sidhu, P.; Cantisani, V.; Dietrich, C.F.; Gilja, O.H.; Saftoiu, A.; Bartels, E.; Bertolotto, M.; Calliada, F.; Clevert, D.A.; Cosgrove, D.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in Non-Hepatic Applications: Update 2017 (Long Version). Ultraschall Med. Eur. J. Ultrasound 2018, 39, e2–e44. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, C.F.; Nolsøe, C.P.; Barr, R.G.; Berzigotti, A.; Burns, P.N.; Cantisani, V.; Chammas, M.C.; Chaubal, N.; Choi, B.I.; Clevert, D.-A.; et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver—Update 2020—WFUMB in Cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Med. Eur. J. Ultrasound 2020, 41, 562–585. [Google Scholar] [CrossRef]
- Piccin, O.; D’Alessio, P.; Cioccoloni, E.; Burgio, L.; Poggi, C.; Altieri, P.; Vicennati, V.; Repaci, A.; Pagotto, U.; Cavicchi, O. Pre-operative imaging workup for surgical intervention in primary hyperparathyroidism: A tertiary referral center experience. Am. J. Otolaryngol. 2021, 42, 102819. [Google Scholar] [CrossRef] [PubMed]
- Noureldine, S.I.; Gooi, Z.; Tufano, R.P. Minimally invasive parathyroid surgery. Gland. Surg. 2015, 4, 410–419. [Google Scholar] [CrossRef]
- Majcen, M.; Hocevar, M. Surgical options in treating patients with primary hyperparathyroidism. Radiol. Oncol. 2020, 54, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, C.F.; Averkiou, M.A.; Correas, J.-M.; Lassau, N.; Leen, E.; Piscaglia, F. An EFSUMB Introduction into Dynamic Contrast-Enhanced Ultrasound (DCE-US) for Quantification of Tumour Perfusion. Ultraschall Med. Eur. J. Ultrasound 2012, 33, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Tranquart, F.; Mercier, L.; Frinking, P.; Gaud, E.; Arditi, M. Perfusion Quantification in Contrast-Enhanced Ultrasound (CEUS)—Ready for Research Projects and Routine Clinical Use. Ultraschall Med. Eur. J. Ultrasound 2012, 33, S31–S38. [Google Scholar] [CrossRef] [PubMed]
- Vitali, F.; Pfeifer, L.; Janson, C.; Goertz, R.; Neurath, M.; Strobel, D.; Wildner, D. Qusantitative perfusion analysis in pancreatic contrast enhanced ultrasound (DCE-US): A promising tool for the differentiation between autoimmune pancreatitis and pancreatic cancer. Z. F R Gastroenterol. 2015, 53, 1175–1181. [Google Scholar] [CrossRef]
- Kuzminski, S.J.; Sosa, J.A.; Hoang, J.K. Update in Parathyroid Imaging. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.; Liang, T.Z.; Grant, E.G.; Swanson, M.S.; Barnett, B. Optimal imaging modality for diagnosis of parathyroid adenoma: Case report and review of the literature. J. Clin. Transl. Endocrinol. Case Rep. 2020, 17, 100065. [Google Scholar] [CrossRef]
- Cakir, B.; Cuhaci, F.N.; Topaloglu, O.; Ozdemir, D.; Dirikoc, A.; Aydin, C.; Polat, S.B.; Ogmen, B.E.; Tam, A.A.; Baser, H.; et al. Ultrasound elastography score and strain index in different parathyroid lesions. Endocr. Connect. 2019, 8, 1579–1590. [Google Scholar] [CrossRef] [Green Version]
- Acar, T.; Ozbek, S.S.; Ertan, Y.; Kavukcu, G.; Tuncyurek, M.; Icoz, R.G.; Akyildiz, M.M.; Makay, O.; Acar, S. Variable sonographic spectrum of parathyroid adenoma with a novel ultrasound finding: Dual concentric echo sign. Med. Ultrason. 2015, 17, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, V.; Sgaramella, L.I.; Di Meo, G.; Prete, F.P.; Logoluso, F.; Minerva, F.; Noviello, M.; Renzulli, G.; Gurrado, A.; Testini, M. Current concepts in parathyroid carcinoma: A single Centre experience. BMC Endocr. Disord. 2019, 19, 46. [Google Scholar] [CrossRef]
- Win, G.M.; Gusov, T.; Marium, F.; Gardner, M.J. Primary Hyperparathyroidism Secondary to a Sestamibi-Negative Cystic Parathyroid Adenoma. Cureus 2021, 13, 17577. [Google Scholar] [CrossRef]
- DeLellis, R.A. Parathyroid tumors and related disorders. Mod. Pathol. 2011, 24, S78–S93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasnicu, C.; Radu, P.A.; Garofil, D.; Bengulescu, J.; Paic, V.; Tigora, A.; Popa, F.; Strambu, V.; Bratucu, M. Clinical Aspects of Parathyroid Hyperplasia in Secondary Renal Hyperparathyroidism. Chirurgia 2019, 114, 594–601. [Google Scholar] [CrossRef]
- Vitetta, G.M.; Ravera, A.; Mensa, G.; Fuso, L.; Neri, P.; Carriero, A.; Cirillo, S. Actual role of color-doppler high-resolution neck ultrasonography in primary hyperparathyroidism: A clinical review and an observational study with a comparison of 99mTc-sestamibi parathyroid scintigraphy. J. Ultrasound 2019, 22, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Bahl, M.; Muzaffar, M.; Vij, G.; Sosa, J.; Choudhury, K.R.; Hoang, J.K. Prevalence of the Polar Vessel Sign in Parathyroid Adenomas on the Arterial Phase of 4D CT. Am. J. Neuroradiol. 2013, 35, 578–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrioli, M.; Valcavi, R. Sonography of Normal and Abnormal Thyroid and Parathyroid Glands. Imaging Endocr. Disord. 2016, 45, 1–15. [Google Scholar] [CrossRef]
- Bunch, P.; Kelly, H. Preoperative Imaging Techniques in Primary Hyperparathyroidism. JAMA Otolaryngol. Neck Surg. 2018, 144, 929–937. [Google Scholar] [CrossRef]
- Palaniappan, M.K. Role of Gray Scale, Color Doppler and Spectral Doppler in Differentiation between Malignant and Benign Thyroid Nodules. J. Clin. Diagn. Res. 2016, 10, TC01–TC06. [Google Scholar] [CrossRef]
- Rohan, K.; Ramesh, A.; Sureshkumar, S.; Vijayakumar, C.; AbdulBasith, K.; Krishnaraj, B. Evaluation of B-Mode and Color Doppler Ultrasound in the Diagnosis of Malignant Cervical Lymphadenopathy. Cureus 2020, 12, 9819. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.P.B.; Jung, E.M.; Jung, F.; Schlitt, H.J.; Hornung, M. VueBox® perfusion analysis of contrast-enhanced ultrasound (CEUS) examinations in patients with primary hyperparathyroidism for preoperative detection of parathyroid gland adenoma. Clin. Hemorheol. Microcirc. 2019, 70, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, P.P.; Hernando, A.S.; Alcalá, B.B.; Rojas-Marcos, P.M.; Catalán, A.L.; Escolá, C. Álvarez Potential Utility of Contrast-Enhanced Ultrasound in the Preoperative Evaluation of Primary Hyperparathyroidism. J. Ultrasound Med. 2019, 38, 2565–2571. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Nie, J.; Zhang, D.; Yang, Q.; Jin, H.; Ou, X.; Ma, X.; Luo, Y. Role of Contrast-Enhanced Ultrasound (CEUS) in the Diagnosis of Cervical Lymph Node Metastasis in Nasopharyngeal Carcinoma (NPC) Patients. Front. Oncol. 2020, 10, 972. [Google Scholar] [CrossRef]
- Cui, X.-W. New ultrasound techniques for lymph node evaluation. World J. Gastroenterol. 2013, 19, 4850–4860. [Google Scholar] [CrossRef]
- Chen, L.; Chen, L.; Liu, J.; Wang, B.; Zhang, H. Value of Qualitative and Quantitative Contrast-Enhanced Ultrasound Analysis in Preoperative Diagnosis of Cervical Lymph Node Metastasis from Papillary Thyroid Carcinoma. J. Ultrasound Med. 2020, 39, 73–81. [Google Scholar] [CrossRef]
- Radzina, M.; Ratniece, M.; Putrins, D.S.; Saule, L.; Cantisani, V. Performance of Contrast-Enhanced Ultrasound in Thyroid Nodules: Review of Current State and Future Perspectives. Cancers 2021, 13, 5469. [Google Scholar] [CrossRef]
- Agha, A.; Hornung, M.; Stroszczynski, C.; Schlitt, H.J.; Jung, E.M. Highly Efficient Localization of Pathological Glands in Primary Hyperparathyroidism Using Contrast-Enhanced Ultrasonography (CEUS) in Comparison with Conventional Ultrasonography. J. Clin. Endocrinol. Metab. 2013, 98, 2019–2025. [Google Scholar] [CrossRef] [Green Version]
- Karakas, E.; Kann, S.; Höffken, H.; Bartsch, D.K.; Celik, I.; Görg, C.; Pfestroff, A. Does Contrast-Enhanced Cervical Ultrasonography Improve Preoperative Localization Results in Patients with Sporadic Primary Hyperparathyroidism? J. Clin. Imaging Sci. 2012, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Hornung, M.; Schlitt, H.J.; Stroszczynski, C.; Jung, E.-M. The role of contrast-enhancend ultrasonography (CEUS) in comparison with 99mTechnetium-sestamibi scintigraphy for localization diagnostic of primary hyperparathyroidism. Clin. Hemorheol. Microcirc. 2014, 58, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, J.; Li, Y.; Wang, H.; Yang, J.; Mou, S.; Zhou, M.; Jiang, C.; Ning, C. The role of preoperative ultrasound, contrast-enhanced ultrasound, and 99mTc-MIBI scanning with single-photon emission computed tomography/X-ray computed tomography localization in refractory secondary hyperparathyroidism. Clin. Hemorheol. Microcirc. 2020, 75, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Sadeghi, R.; Schalin-Jäntti, C.; Caldarella, C.; Ceriani, L.; Giovanella, L.; Eisele, D.W. Detection rate of 99m Tc-MIBI single photon emission computed tomography (SPECT)/CT in preoperative planning for patients with primary hyperparathyroidism: A meta-analysis. Head Neck 2016, 38, E2159–E2172. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Number of Patients (n = 75) |
|---|---|
| Age (years), mean (range) | 59.8 (19–82) |
| Female | 64 (85.3%) |
| Male | 11 (14.7%) |
| Clinical data | |
| 39 (52.0%) |
| 25 (33.3%) |
| 23 (30.6%) |
| Morphology | Number of lesions (n = 88) |
| 71 (80.7%) |
| 17 (19.3%) |
| Characteristics | Number of patients (lesions) |
| Single lesion: | 67 (67) |
| 60 (60) |
| 7 (7) |
| Multiple lesions | 8 (21) |
| 4 (8) |
| 2 (4) |
| 1 (4) |
| 1 (5) |
| Total: 75 (88) |
| Characteristics | Adenoma (n = 70) | Hyperplasia (n = 12) | p-Value |
|---|---|---|---|
| Median (range), mm | 13 (3–43) | 10 (2–30) | 0.995 |
| Median volume (range), mm3 | 955 (65–8190) | 364 (52–4680) | 0.039 |
| <1000 mm3—n (%) | 36 (51.4%) | 10 (80%) | |
| ≥1000 mm3—n (%) | 34 (48.6%) | 2 (20%) | |
| Hyperechoic central part | 31 (44.3%) | 6 (50%) | 0.717 |
| Cystic component | 10 (14.3%) | 7 (58.2%) | 0.001 |
| Polar vessel | 70 (100%) | 10 (83.3%) | 0.565 |
| Vascularisation pattern | |||
| Central | 8 (11.4%) | 1 (8.3%) | |
| Peripheral | 19 (27.1%) | 2 (16.7%) | 0.924 |
| Combined | 33 (47.2%) | 8 (66.7%) | |
| Absent | 10 (14.3%) | 1 (8.3%) | |
| Median (range) | |||
| The onset of contrast uptake (s) | 11 (4–20) | 12 (7–20) | 0.714 |
| Time of maximum contrast concentration (s) | 16 (8–30) | 18 (11–28) | 0.067 |
| The onset of contrast wash-out (s) | 27 (12–120) | 25 (18–120) | 0.553 |
| Serum parathormone, pg/mL | 162 (74–1166) | 186 (90–1360) | 0.026 |
| Serum calcium, mmol/L | 2.8 (2.3–4.2) | 2.7 (2.6–3.1) | 0.922 |
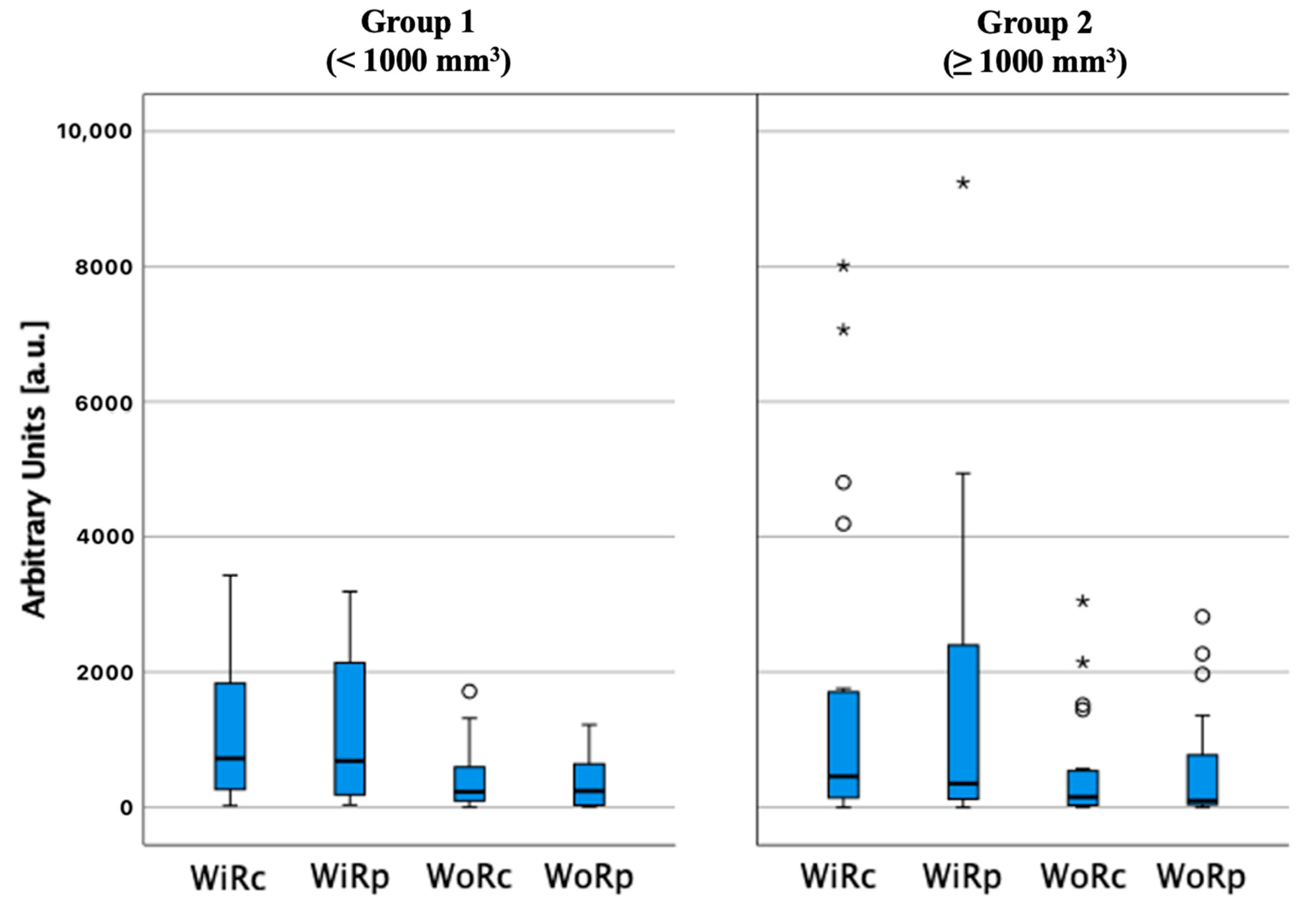
| Group 1 | Group 2 | p-Value | |
|---|---|---|---|
| <1000 m3 (n = 28) | ≥1000 mm3 (n = 32) | ||
| Median (Range) | Median (Range) | ||
| Onset of contrast uptake (s) | 11 (8–20) | 12 (7–18) | 0.901 |
| Time of maximum contrast concentration (s) | 16 (12–30) | 18.8 (9–30) | 0.341 |
| The onset of contrast | 27 (15–120) | 29 (15–120) | 0.376 |
| wash-out (s) | |||
| Serum parathormone, pg/mL | 131 (74–363) | 182 (84–1059) | 0.005 |
| Serum calcium, mmol/L | 2.7 (2.5–3.3) | 2.8 (2.4–4.2) | 0.199 |
| 1 mL | 2 mL | pValue | |||
| Mean | 95%-CI | Mean | 95%-CI | ||
| ACSIc (a.u.) | 1756 | 396–3174 | 1720 | 436–3005 | 0.386 |
| ACSIp (a.u.) | 1785 | 539–2613 | 1778 | 679–2877 | 0.436 |
| WiRc (a.u.) | 1146 | 530–2363 | 1474 | 684–2264 | 0.402 |
| WiRp (a.u.) | 1423 | 538–2308 | 1369 | 599–2140 | 0.436 |
| WoRc (a.u.) | 535 | 187–884 | 530 | 258–801 | 0.631 |
| WoRp (a.u.) | 532 | 173–892 | 471 | 208–734 | 0.356 |
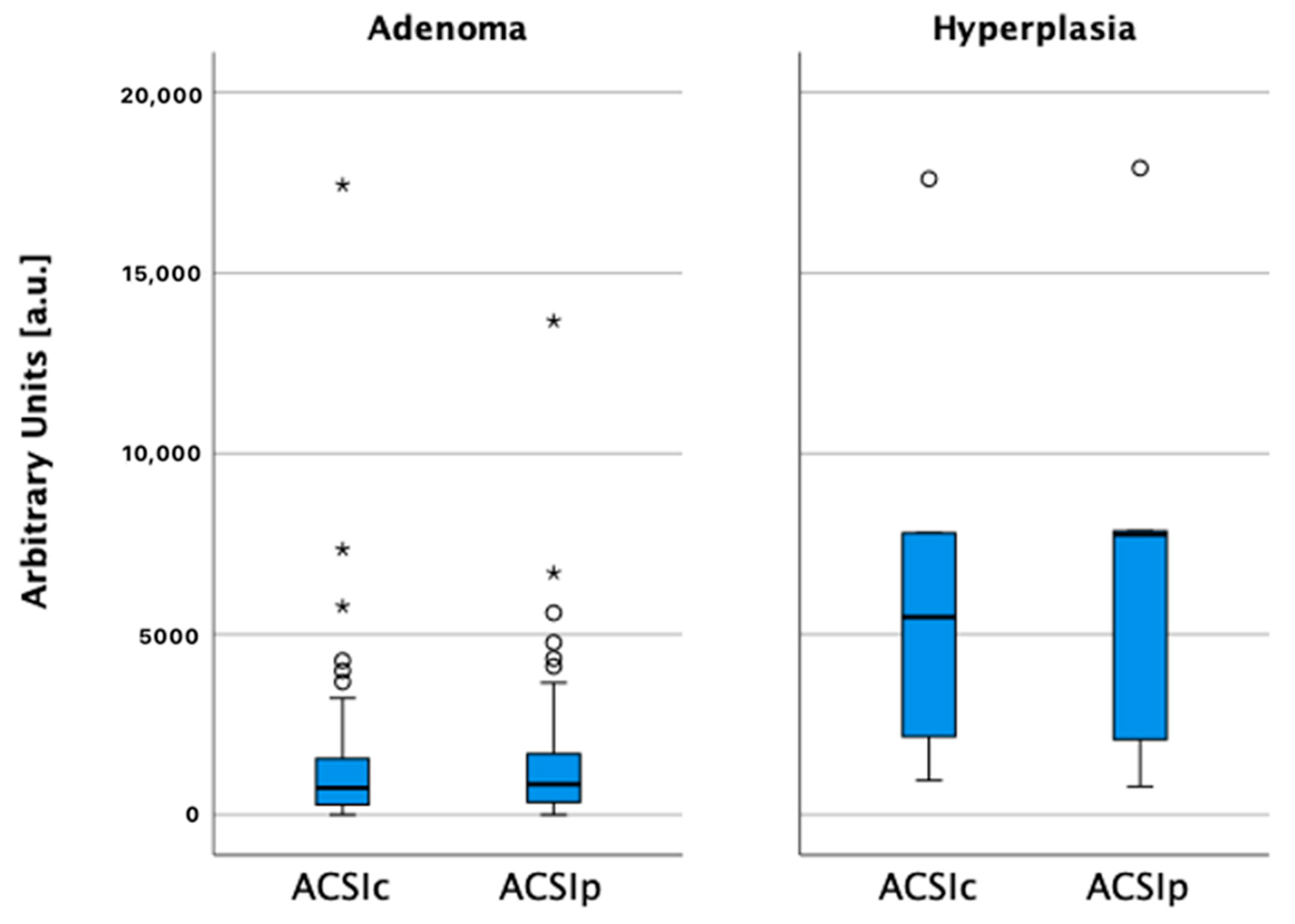
| Adenoma | Hyperplasia | p-Value | |||
|---|---|---|---|---|---|
| Mean | 95%-CI | Mean | 95%-CI | ||
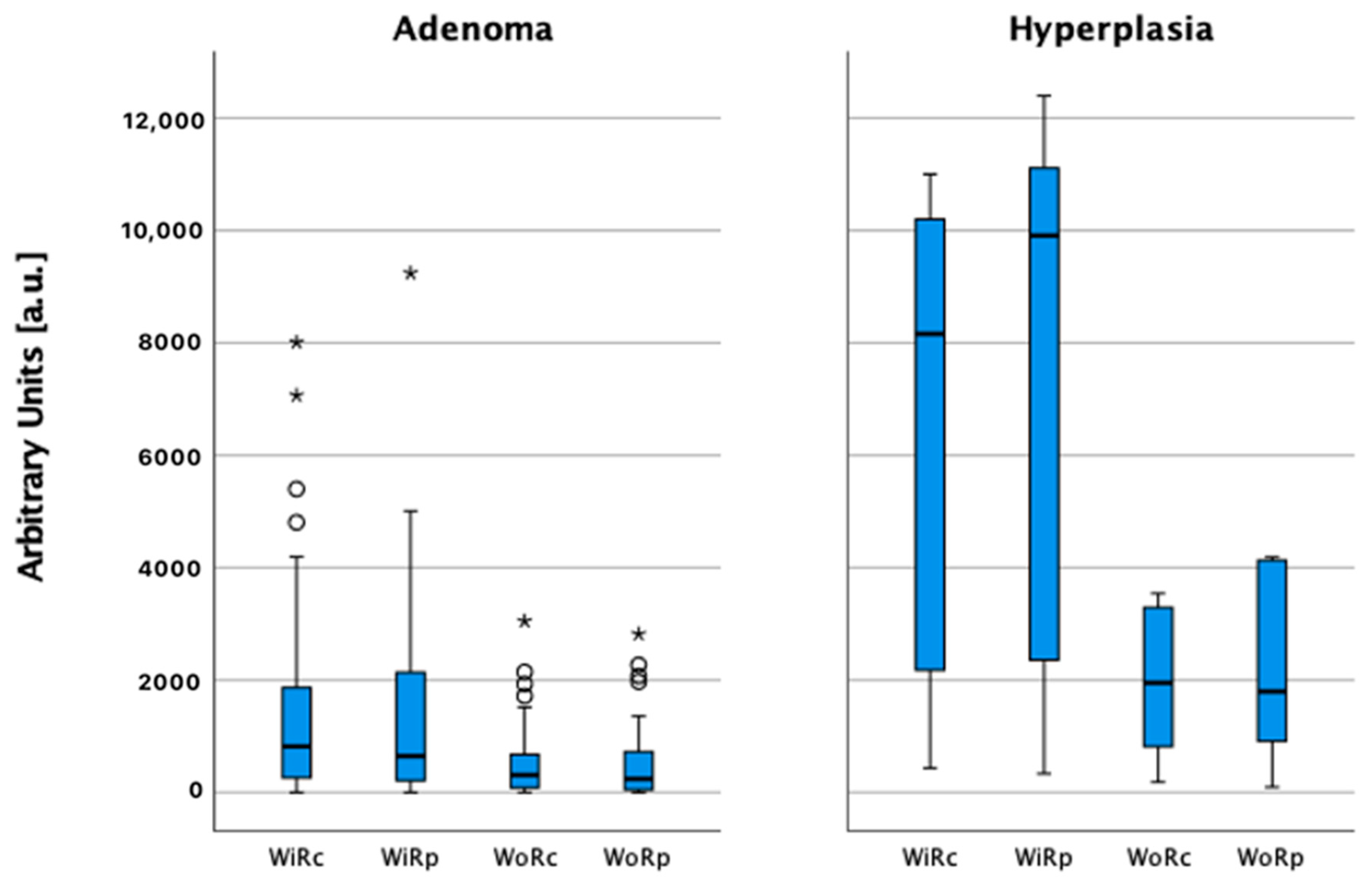
| ACSIc (a.u.) | 1638 | 779–2366 | 6797 | 1416–15012 | 0.002 |
| ACSIp (a.u.) | 1646 | 909–2512 | 7275 | 1111–15662 | <0.001 |
| WiRc (a.u.) | 1502 | 924–2081 | 8160 | 431–12356 | <0.001 |
| WiRp (a.u.) | 1445 | 871–2019 | 7222 | 412–14031 | <0.001 |
| WoRc (a.u.) | 558 | 345–770 | 1956 | 123–1956 | <0.001 |
| WoRp (a.u.) | 552 | 310–735 | 2224 | 92–4542 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlovics, S.; Radzina, M.; Niciporuka, R.; Ratniece, M.; Mikelsone, M.; Tauvena, E.; Liepa, M.; Prieditis, P.; Ozolins, A.; Gardovskis, J.; et al. Contrast-Enhanced Ultrasound Qualitative and Quantitative Characteristics of Parathyroid Gland Lesions. Medicina 2022, 58, 2. https://doi.org/10.3390/medicina58010002
Pavlovics S, Radzina M, Niciporuka R, Ratniece M, Mikelsone M, Tauvena E, Liepa M, Prieditis P, Ozolins A, Gardovskis J, et al. Contrast-Enhanced Ultrasound Qualitative and Quantitative Characteristics of Parathyroid Gland Lesions. Medicina. 2022; 58(1):2. https://doi.org/10.3390/medicina58010002
Chicago/Turabian StylePavlovics, Sergejs, Maija Radzina, Rita Niciporuka, Madara Ratniece, Madara Mikelsone, Elina Tauvena, Mara Liepa, Peteris Prieditis, Arturs Ozolins, Janis Gardovskis, and et al. 2022. "Contrast-Enhanced Ultrasound Qualitative and Quantitative Characteristics of Parathyroid Gland Lesions" Medicina 58, no. 1: 2. https://doi.org/10.3390/medicina58010002
APA StylePavlovics, S., Radzina, M., Niciporuka, R., Ratniece, M., Mikelsone, M., Tauvena, E., Liepa, M., Prieditis, P., Ozolins, A., Gardovskis, J., & Narbuts, Z. (2022). Contrast-Enhanced Ultrasound Qualitative and Quantitative Characteristics of Parathyroid Gland Lesions. Medicina, 58(1), 2. https://doi.org/10.3390/medicina58010002






