Three-Dimensional Transesophageal Echocardiography in the Diagnosis and Treatment of Mitral Prosthetic Valve Endocarditis—A Narrative Review
Abstract
:1. Introduction
2. D TEE vs. 3D TEE in Mitral PVE Treatment
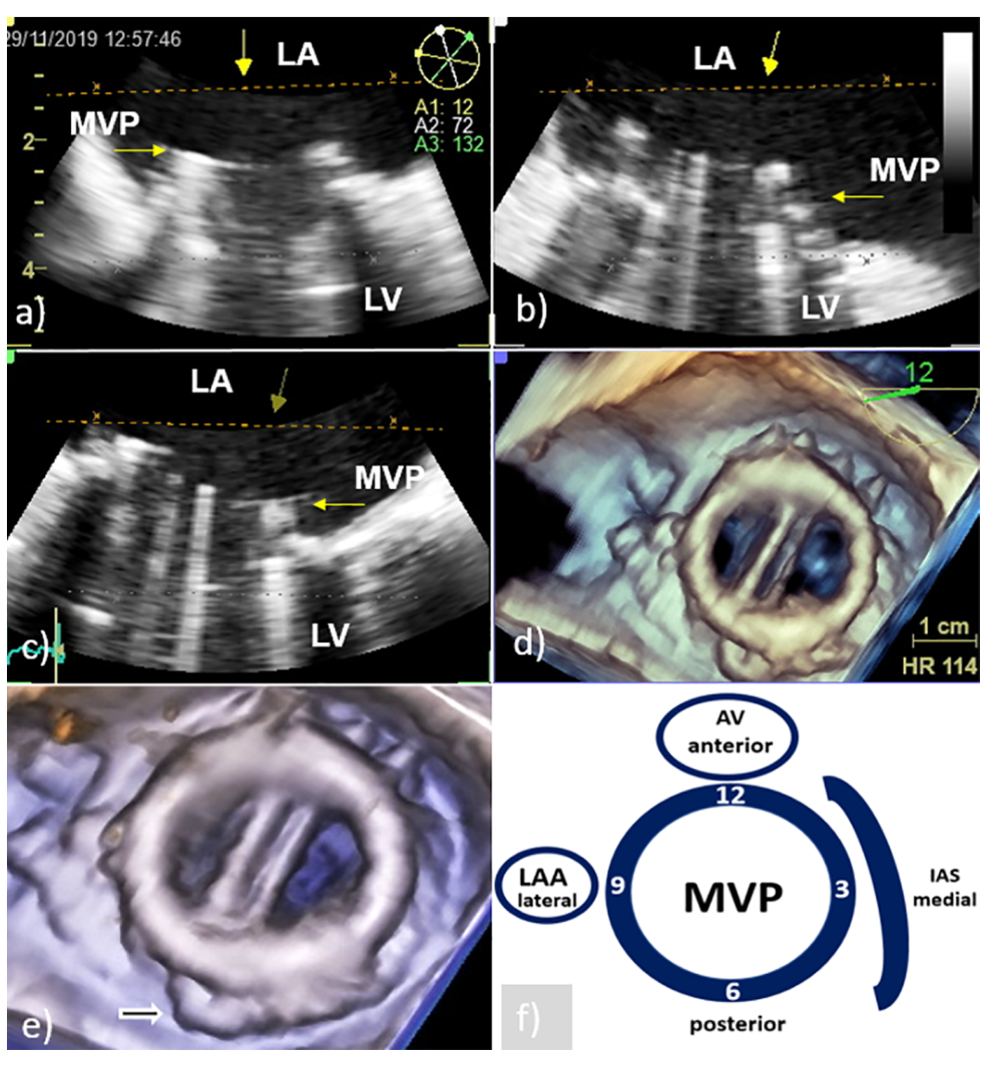
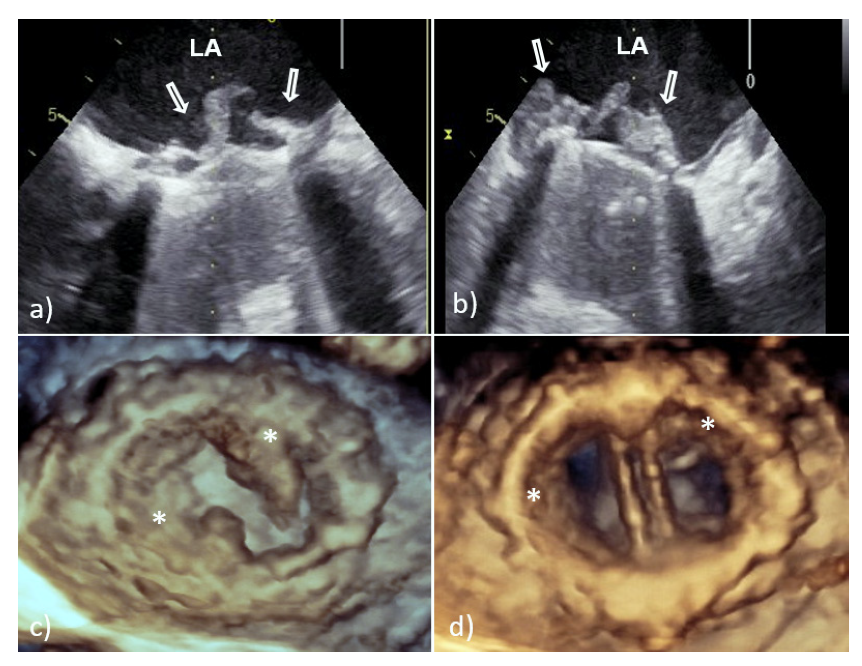
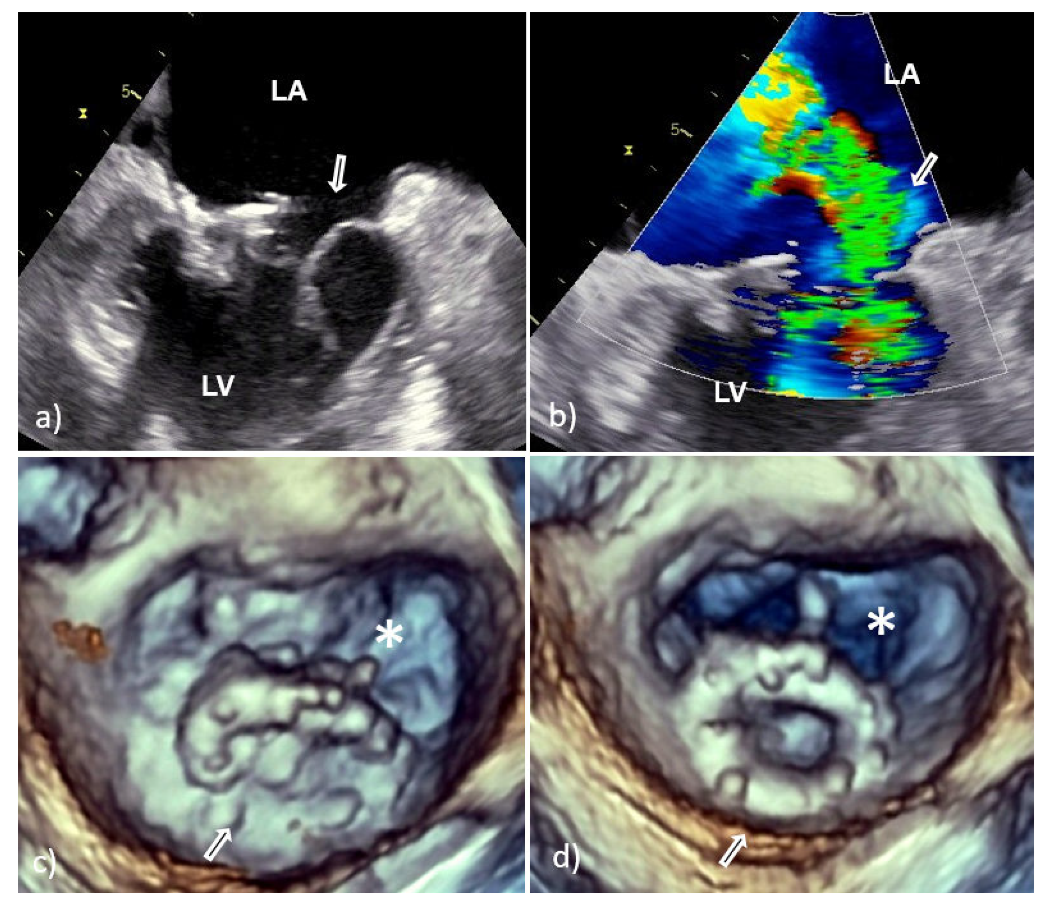
3. Echocardiographic Presentation of the Mitral PVE
- oscillating intracardiac mass in accordance with vegetation,
- abscess formation,
- presence of paravalvular regurgitation/leakage or dehiscence of prosthetic valve.
3.1. PVE Vegetation
3.2. Perivalvular Complications
4. Advantages and Limitations of 3D TEE in PVE Diagnostics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Habib, G.; Thuny, F.; Avgerinos, J.F. Prosthetic valve endocarditis: Current approach and therapeutic options. Prog. Cardiovasc. Dis. 2008, 50, 274–281. [Google Scholar] [CrossRef]
- Chirillo, F.; Scotto, P.; Rocco, F.; Rigoli, R.; Pedrocco, A.; Martire, P. Management strategies and outcome for prosthetic valve endocarditis. Am. J. Cardiol. 2013, 112, 1177–1181. [Google Scholar] [CrossRef]
- Wang, A.; Athan, E.; Pappas, P.A.; Fowler, V.G., Jr.; Olaison, L.; Paré, C.; Almirante, B.; Muñoz, P.; Rizzi, M.; Naber, C.; et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007, 297, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, A.; Sohail, M.R.; Steckelberg, J.M. Prosthetic valve endocarditis: State of the heart. Clin. Investig. 2012, 2, 803–817. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef] [PubMed]
- Afonso, L.; Kottam, A.; Reddy, V.; Penumetcha, A. Echocardiography in infective endocarditis: State of the art. Curr. Cardiol. Rep. 2017, 19, 127. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Tong, A.T. Role of echocardiography in the diagnosis and management of infective endocarditis. Curr. Opin. Cardiol. 2002, 17, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.M.; Nosir, Y.F.; Alasnag, M.; Chamsi-Pasha, H. Real time three-dimensional transesophageal echocardiography: A novel approach for the assessment of prosthetic heart valves. Echocardiography 2014, 31, 188–196. [Google Scholar] [CrossRef]
- Bai, A.D.; Steinberg, M.; Showler, A.; Burry, L.; Bhatia, R.S.; Tomlinson, G.A.; Bell, C.M.; Morris, A.M. Diagnostic accuracy of transthoracic echocardiography for infective endocarditis findings using transesophageal echocardiography as the reference standard: A meta-analysis. J. Am. Soc. Echocardiogr. 2017, 30, 639–646.e8. [Google Scholar] [CrossRef] [PubMed]
- Berdejo, J.; Shibayama, K.; Harada, K.; Tanaka, J.; Mihara, H.; Gurudevan, S.V.; Siegel, R.J.; Shiota, T. Evaluation of vegetation size and its relationship with embolism in infective endocarditis: A real-time 3-dimensional transesophageal echocardiography study. Circ. Cardiovasc. Imaging 2014, 7, 149–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansalia, S.; Biswas, M.; Dutta, R.; Hage, F.G.; MCHsiung Nanda, N.C.; Singh, P.; Manda, J.; Kesanolla, S.K.; Wei, J.; Yin, W.-H. The value of live/real time three-dimensional transesophageal echocardiography in the assessment of valvular vegetation. Echocardiography 2009, 26, 1264–1273. [Google Scholar] [CrossRef]
- Galzerano, D.; Kinsara, A.J.; Di Michele, S.; Vriz, O.; Fadel, B.M.; Musci, R.L.; Galderisi, M.; Al Sergani, H.; Colonna, P. Three dimensional transesophageal echocardiography: A missing link in infective endocarditis imaging? Int. J. Cardiovasc. Imaging 2020, 36, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud-Elsayed, H. Added value of three-dimensional transesophageal echocardiography in management of mitral paravalvular leaks. Echocardiography 2020, 37, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Quader, N.; Rigolin, V.H. Two and three dimensional echocardiography for pre-operative assessment of mitral valve regurgitation. Cardiovasc. Ultrasound 2014, 12, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durak, D.T.; Lukes, A.S.; Bright, D.K. New criteria for diagnosis of infective endocarditis: Utilization of specific echocardiographic findings. Duke Endocarditis Service. Am. J. Med. 1994, 96, 200–209. [Google Scholar] [CrossRef]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Matsukuma, S.; Eishi, K.; Tanigawa, K.; Miura, T.; Matsumaru, I.; Hisatomi, K.; Tsuneto, A. Afebrile pannus-induced blood culture-negative mechanical valve endocarditis. Ann. Thorac. Surg. 2016, 102, e511–e513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ya’qoub, L.; Eng, M.H. Bioprosthetic valve infective endocarditis: Why is it important? Heart 2020, 106, 1378–1379. [Google Scholar] [CrossRef] [PubMed]
- Tsang, W.; Weinert, L.; Kronzon, I.; Lang, R.M. Three-dimensional echocardiography in the assessment of prosthetic valves. Rev. Esp. Cardiol. 2011, 64, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Erba, P.A.; Pizzi, M.N.; Roque, A.; Salaun, E.; Lancellotti, P.; Tornos, P.; Habib, G. Multimodality imaging in infective endocarditis: An imaging team within the endocarditis team. Circulation 2019, 140, 1753–1765. [Google Scholar] [CrossRef] [PubMed]


| 3D TEE vs. 2D TEE | |
|---|---|
| PV vegetation | |
| Identification | superior |
| number | superior |
| size | superior |
| location | superior |
| attachment | superior |
| PV abscess | |
| identification | equal |
| size | superior |
| site | superior |
| extension | superior |
| communication | superior |
| PV regurgitation | |
| transvalvular | equal |
| paravalvular | superior |
| mechanism | superior |
| severity | equal, both satisfying |
| jet direction | equal, both satisfying |
| PV dehiscence | |
| identification | superior |
| size | superior |
| site | superior |
| shape | superior |
| area | superior |
| relation to leak | superior |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carević, V.; Mladenović, Z.; Perković-Avelini, R.; Bečić, T.; Radić, M.; Fabijanić, D. Three-Dimensional Transesophageal Echocardiography in the Diagnosis and Treatment of Mitral Prosthetic Valve Endocarditis—A Narrative Review. Medicina 2022, 58, 23. https://doi.org/10.3390/medicina58010023
Carević V, Mladenović Z, Perković-Avelini R, Bečić T, Radić M, Fabijanić D. Three-Dimensional Transesophageal Echocardiography in the Diagnosis and Treatment of Mitral Prosthetic Valve Endocarditis—A Narrative Review. Medicina. 2022; 58(1):23. https://doi.org/10.3390/medicina58010023
Chicago/Turabian StyleCarević, Vedran, Zorica Mladenović, Ružica Perković-Avelini, Tina Bečić, Mislav Radić, and Damir Fabijanić. 2022. "Three-Dimensional Transesophageal Echocardiography in the Diagnosis and Treatment of Mitral Prosthetic Valve Endocarditis—A Narrative Review" Medicina 58, no. 1: 23. https://doi.org/10.3390/medicina58010023
APA StyleCarević, V., Mladenović, Z., Perković-Avelini, R., Bečić, T., Radić, M., & Fabijanić, D. (2022). Three-Dimensional Transesophageal Echocardiography in the Diagnosis and Treatment of Mitral Prosthetic Valve Endocarditis—A Narrative Review. Medicina, 58(1), 23. https://doi.org/10.3390/medicina58010023







