Acute Presentation of Large Size Clear Cell Ovarian Carcinoma as Double Torsed Ovarian Tumor
Abstract
:1. Introduction
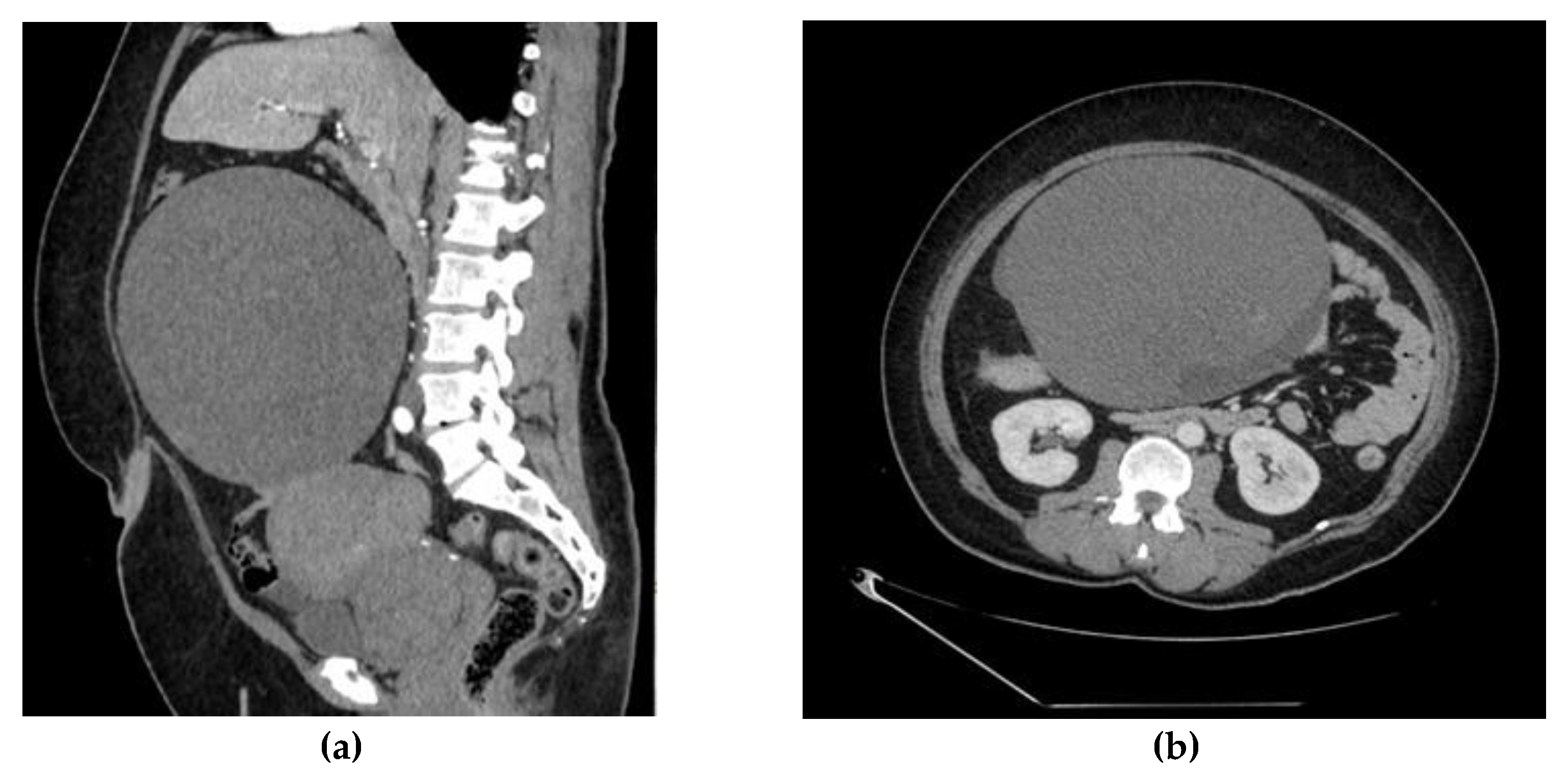
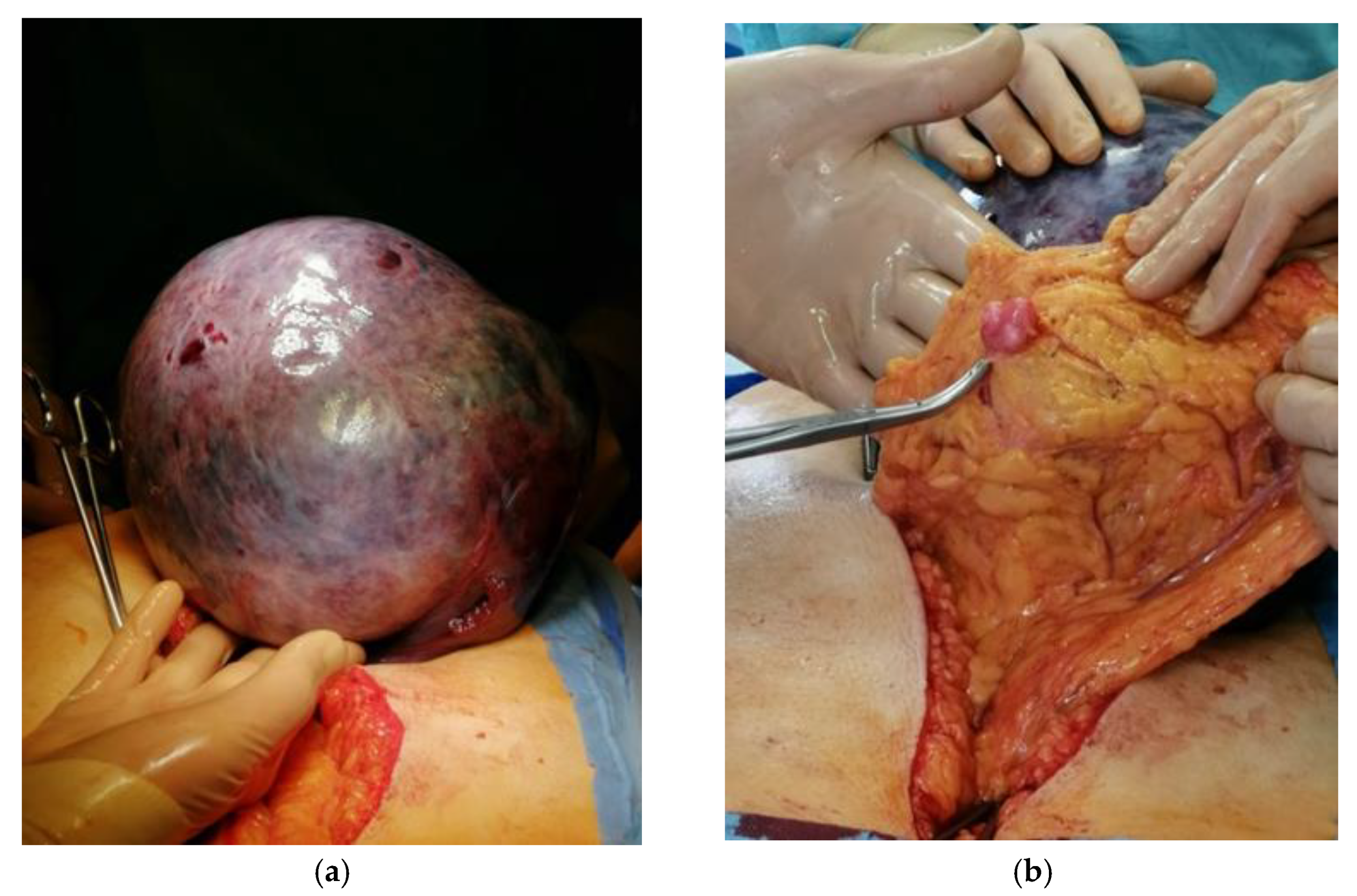
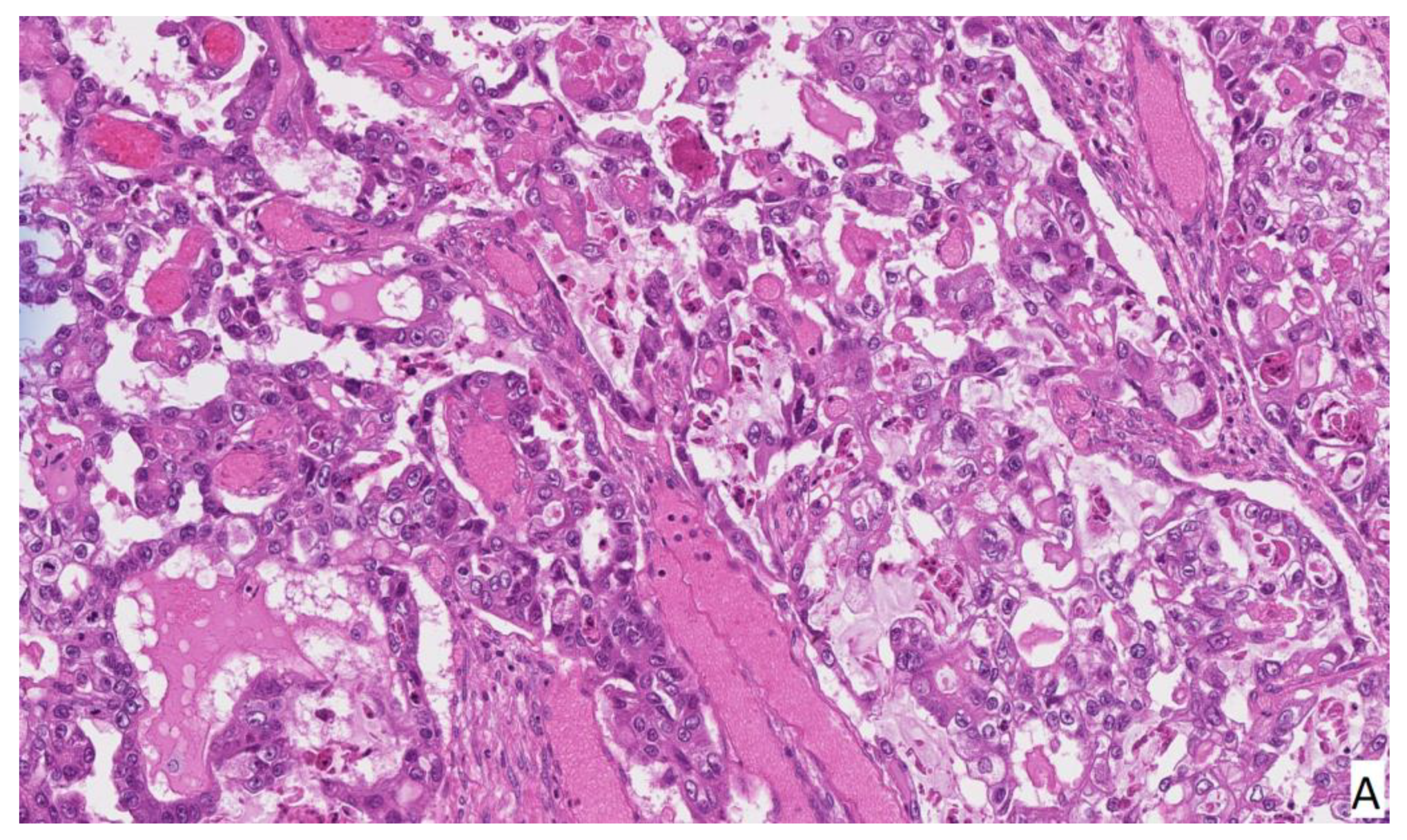
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef] [Green Version]
- Yuk, J.-S.; Kim, L.Y.; Shin, J.-Y.; Choi, D.Y.; Kim, T.Y.; Lee, J.H. A national population-based study of the incidence of adnexal torsion in the Republic of Korea. Int. J. Gynecol. Obstet. 2015, 129, 169–170. [Google Scholar] [CrossRef]
- Huang, C.; Hong, M.-K.; Ding, D.-C. A review of ovary torsion. Tzu-chi Med. J. 2017, 29, 143–147. [Google Scholar]
- Desai, A.; Xu, J.; Aysola, K.; Qin, Y.; Okoli, C.; Hariprasad, R.; Chinemerem, U.; Gates, C.; Reddy, A.; Danner, O.; et al. Epithelial ovarian cancer: An overview. World J. Transl. Med. 2014, 3, 1–8. [Google Scholar] [CrossRef]
- Nik, N.N.; Vang, R.; Shih, I.-M.; Kurman, R.J. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu. Rev. Pathol. 2014, 9, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Oseledchyk, A.; Leitao, M.M.; Konner, J.; O’Cearbhaill, R.E.; Zamarin, D.; Sonoda, Y.; Gardner, G.J.; Roche, K.L.; Aghajanian, C.A.; Grisham, R.N.; et al. Adjuvant chemotherapy in patients with stage I endometrioid or clear cell ovarian cancer in the platinum era: A Surveillance, Epidemiology, and End Results Cohort Study, 2000–2013. Ann. Oncol. 2017, 28, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Shintani, D.; Nishikawa, T. Clear-cell carcinoma of the ovary. Ann. Oncol. 2016, 27, i50–i52. [Google Scholar] [CrossRef]
- Pearce, C.L.; Templeman, C.; Rossing, M.A.; Lee, A.; Near, A.M.; Webb, P.M.; Nagle, C.M.; Doherty, J.A.; Cushing-Haugen, K.L.; Wicklund, K.G.; et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case–control studies. Lancet Oncol. 2012, 13, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Kim, M.A.; Lee, M.; Suh, D.H.; Kim, K.; No, J.H.; Chung, H.H.; Kim, Y.B.; Song, Y.S. Effect of Endometriosis on the Prognosis of Ovarian Clear Cell Carcinoma: A Two-Center Cohort Study and Meta-analysis. Ann. Surg. Oncol. 2015, 22, 2738–2745. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Ditto, A.; Lopez, S.; Bertolina, F.; Murgia, F.; Pinelli, C.; Chiappa, V.; Raspagliesi, F. Adjuvant chemotherapy vs. observation in stage I clear cell ovarian carcinoma: A systematic review and meta-analysis. Gynecol. Oncol. 2020, 157, 293–298. [Google Scholar] [CrossRef]
- Ratnavelu, N.D.; Brown, A.P.; Mallett, S.; Scholten, R.J.; Patel, A.; Founta, C.; Galaal, K.; Cross, P.; Naik, R. Intraoperative frozen section analysis for the diagnosis of early stage ovarian cancer in suspicious pelvic masses. Cochrane Database Syst. Rev. 2016, CD010360. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.; Haldar, K.; Kehoe, S.; Lawrie, T.A. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database Syst. Rev. 2012, CD005343. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kajiyama, H.; Shibata, K.; Ino, K.; Nawa, A.; Sakakibara, K.; Matsuzawa, K.; Takeda, A.; Kinoshita, Y.; Kawai, M.; et al. Is there any association between retroperitoneal lymphadenectomy and survival benefit in ovarian clear cell carcinoma patients? Ann. Oncol. 2008, 19, 1284–1287. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Munro, E.G.; Cheung, M.K.; Husain, A.; Teng, N.N.; Berek, J.S.; Osann, K. Association of Lymphadenectomy and Survival in Stage I Ovarian Cancer Patients. Obstet. Gynecol. 2007, 109, 12–19. [Google Scholar] [CrossRef]
- Wiggans, A.J.; Cass, G.K.; Bryant, A.; Lawrie, T.A.; Morrison, J. Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer. Cochrane Database Syst. Rev. 2015, CD007929. [Google Scholar] [CrossRef]
- Arians, N.; Kieser, M.; Benner, L.; Rochet, N.; Katayama, S.; Sterzing, F.; Herfarth, K.; Schubert, K.; Schröder, L.; Leitzen, C.; et al. Adjuvant Intensity Modulated Whole-Abdominal Radiation Therapy for High-Risk Patients with Ovarian Cancer (International Federation of Gynecology and Obstetrics Stage III): First Results of a Prospective Phase 2 Study. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 912–920. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bužinskienė, D.; Rudaitis, V.; Misevičiūtė, K. Acute Presentation of Large Size Clear Cell Ovarian Carcinoma as Double Torsed Ovarian Tumor. Medicina 2022, 58, 89. https://doi.org/10.3390/medicina58010089
Bužinskienė D, Rudaitis V, Misevičiūtė K. Acute Presentation of Large Size Clear Cell Ovarian Carcinoma as Double Torsed Ovarian Tumor. Medicina. 2022; 58(1):89. https://doi.org/10.3390/medicina58010089
Chicago/Turabian StyleBužinskienė, Diana, Vilius Rudaitis, and Karolina Misevičiūtė. 2022. "Acute Presentation of Large Size Clear Cell Ovarian Carcinoma as Double Torsed Ovarian Tumor" Medicina 58, no. 1: 89. https://doi.org/10.3390/medicina58010089
APA StyleBužinskienė, D., Rudaitis, V., & Misevičiūtė, K. (2022). Acute Presentation of Large Size Clear Cell Ovarian Carcinoma as Double Torsed Ovarian Tumor. Medicina, 58(1), 89. https://doi.org/10.3390/medicina58010089





