Serum Procalcitonin Levels in Newly Diagnosed Hodgkin Lymphoma: Correlation with Other Inflammatory Biomarkers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Staging
2.2. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Serum Inflammatory Marker Levels
3.2. Serum PCT Levels
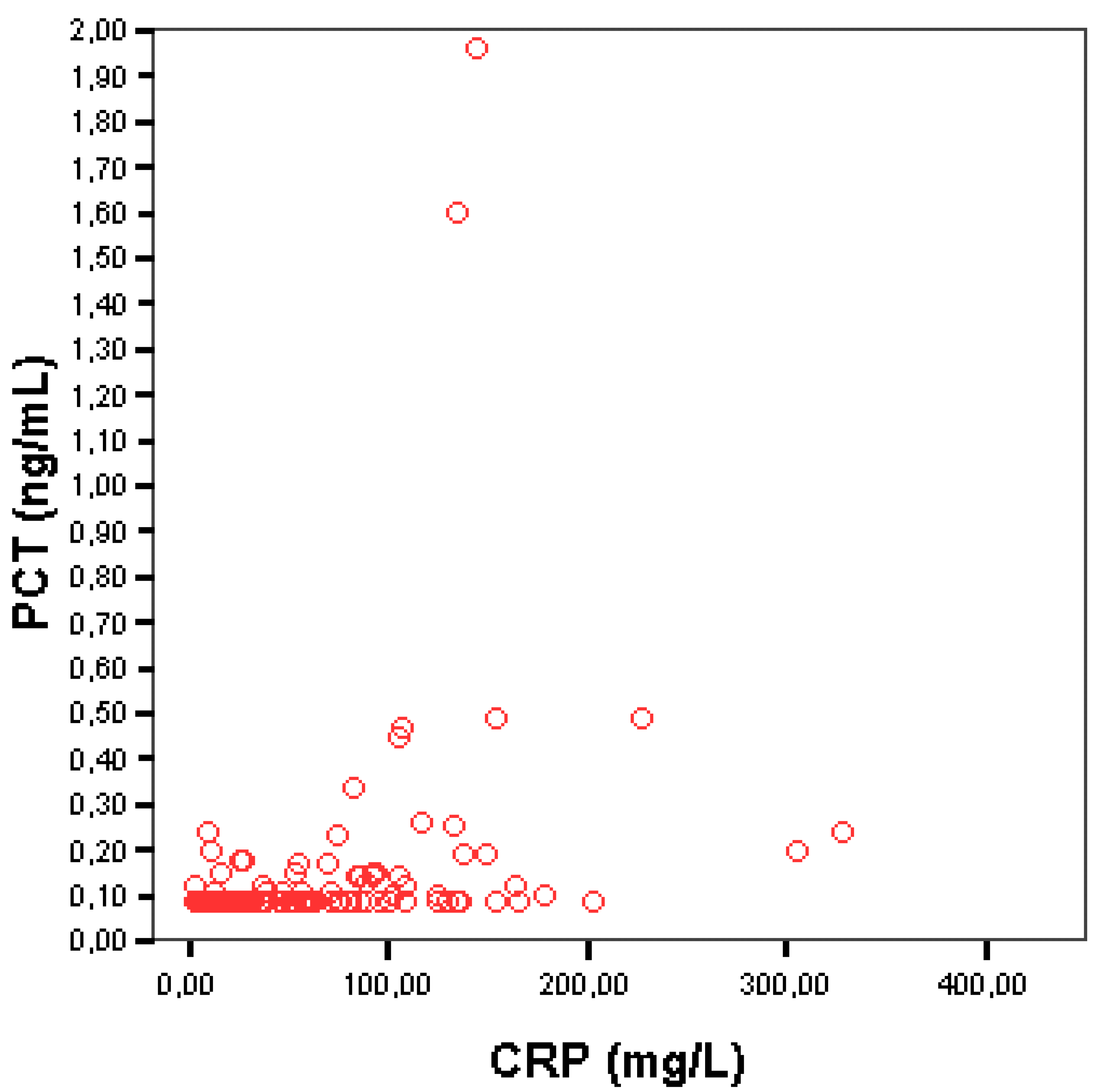
3.3. Correlation between Serum PCT and CRP
3.4. Correlation between Serum PCT and Other Inflammatory and Non-Inflammatory Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Covington, E.W.; Roberts, M.Z.; Dong, J. Procalcitonin Monitoring as a Guide for Antimicrobial Therapy: A Review of Current Literature. Pharmacotherapy 2018, 38, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.L.; Nylén, E.S.; White, J.C.; Müller, B.; Snider, R.H. Clinical Review 167: Procalcitonin and the Calcitonin Gene Family of Peptides in Inflammation, Infection, and Sepsis: A Journey from Calcitonin Back to Its Precursors. J. Clin. Endocrinol. Metab. 2004, 89, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Maruna, P.; Nedělníková, K.; Gürlich, R. Physiology and Genetics of Procalcitonin. Physiol. Res. 2000, 49 (Suppl. S1), S57–S61. [Google Scholar] [PubMed]
- Lippi, G.; Sanchis-Gomar, F. Procalcitonin in Inflammatory Bowel Disease: Drawbacks and Opportunities. World J. Gastroenterol. 2017, 23, 8283–8290. [Google Scholar] [CrossRef] [PubMed]
- Soreng, K.; Levy, H.R. Procalcitonin: An Emerging Biomarker of Bacterial Sepsis. Clin. Microbiol. Newsl. 2011, 33, 171–178. [Google Scholar] [CrossRef]
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J. Diagnostic Value of Procalcitonin, Interleukin-6, and Interleukin-8 in Critically Ill Patients Admitted with Suspected Sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402. [Google Scholar] [CrossRef]
- Nakamura, M.; Kono, R.; Nomura, S.; Utsunomiya, H. Procalcitonin: Mysterious Protein in Sepsis. J. Basic Clin. Med. 2013, 2, 7–11. [Google Scholar]
- Tujula, B.; Hämäläinen, S.; Kokki, H.; Pulkki, K.; Kokki, M. Review of Clinical Practice Guidelines on the Use of Procalcitonin in Infections. Infect. Dis. 2020, 52, 227–234. [Google Scholar] [CrossRef]
- Becker, K.L.; Snider, R.; Nylen, E.S. Procalcitonin Assay in Systemic Inflammation, Infection, and Sepsis: Clinical Utility and Limitations. Crit. Care Med. 2008, 36, 941–952. [Google Scholar] [CrossRef]
- Schuetz, P.; Maurer, P.; Punjabi, V.; Desai, A.; Amin, D.N.; Gluck, E. Procalcitonin Decrease over 72 Hours in US Critical Care Units Predicts Fatal Outcome in Sepsis Patients. Crit. Care 2013, 17, R115. [Google Scholar] [CrossRef]
- Müller, B.; Becker, K.L.; Schächinger, H.; Rickenbacher, P.R.; Huber, P.R.; Zimmerli, W.; Ritz, R. Calcitonin Precursors Are Reliable Markers of Sepsis in a Medical Intensive Care Unit. Crit. Care Med. 2000, 28, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Meisner, M. Update on Procalcitonin Measurements. Ann. Lab. Med. 2014, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Vassilakopoulos, T.P.; Angelopoulou, M.K.; Siakantaris, M.P.; Kontopidou, F.N.; Dimopoulou, M.N.; Barbounis, A.; Grigorakis, V.; Karkantaris, C.; Anargyrou, K.; Chatziioannou, M.; et al. Prognostic Factors in Advanced Stage Hodgkin’s Lymphoma: The Significance of the Number of Involved Anatomic Sites. Eur. J. Haematol. 2001, 67, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Hasenclever, D.; Diehl, V.; Armitage, J.O.; Assouline, D.; Björkholm, M.; Brusamolino, E.; Canellos, G.P.; Carde, P.; Crowther, D.; Cunningham, D.; et al. A Prognostic Score for Advanced Hodgkin’s Disease. N. Engl. J. Med. 1998, 339, 1506–1514. [Google Scholar] [CrossRef]
- Karakatsanis, S.; Panitsas, F.; Arapaki, M.; Galopoulos, D.; Asimakopoulos, J.V.; Liaskas, A.; Chatzidimitriou, C.; Belia, M.; Konstantinou, E.; Vassilopoulos, I.; et al. Serum Ferritin Levels in Previously Untreated Classical Hodgkin Lymphoma: Correlations and Prognostic Significance. Leuk. Lymphoma 2022, 63, 799–812. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.; Pappi, V.; Constantinou, N.; Yiakoumis, X.; Tsorra, O.; Dimou, M.; Konstantinidou, P.; Pessach, I.; Moschoyiannis, M.; Bartzi, V.; et al. Baseline Serum C-Reactive Protein Levels (CRP) in Hodgkin Lymphoma (HL): Correlations with Clinical and Laboratory Parameters and Prognostic Significance under Anthracycline-Based Chemotherapy. In Proceedings of the 16th Congress of the European Haematology Association, London, UK, 9–12 June 2011; Volume 96, p. 318. [Google Scholar]
- Vassilakopoulos, T.P.; Angelopoulou, M.K.; Constantinou, N.; Karmiris, T.; Repoussis, P.; Roussou, P.; Siakantaris, M.P.; Korkolopoulou, P.; Kyrtsonis, M.C.; Kokoris, S.I.; et al. Development and Validation of a Clinical Prediction Rule for Bone Marrow Involvement in Patients with Hodgkin Lymphoma. Blood 2005, 105, 1875–1880. [Google Scholar] [CrossRef]
- Engert, A.; Plütschow, A.; Eich, H.T.; Lohri, A.; Dörken, B.; Borchmann, P.; Berger, B.; Greil, R.; Willborn, K.C.; Wilhelm, M.; et al. Reduced Treatment Intensity in Patients with Early-Stage Hodgkin’s Lymphoma. N. Engl. J. Med. 2010, 363, 640–652. [Google Scholar] [CrossRef]
- Eich, H.T.; Diehl, V.; Görgen, H.; Pabst, T.; Markova, J.; Debus, J.; Ho, A.; Dörken, B.; Rank, A.; Grosu, A.L.; et al. Intensified Chemotherapy and Dose-Reduced Involved-Field Radiotherapy in Patients with Early Unfavorable Hodgkin’s Lymphoma: Final Analysis of the German Hodgkin Study Group HD11 Trial. J. Clin. Oncol. 2010, 28, 4199–4206. [Google Scholar] [CrossRef]
- Hohaus, S.; Massini, G.; Giachelia, M.; Vannata, B.; Bozzoli, V.; Cuccaro, A.; D’Alo’, F.; Larocca, L.M.; Raymakers, R.A.P.; Swinkels, D.W.; et al. Anemia in Hodgkin’s Lymphoma: The Role of Interleukin-6 and Hepcidin. J. Clin. Oncol. 2010, 28, 2538–2543. [Google Scholar] [CrossRef]
- Koliaraki, V.; Marinou, M.; Vassilakopoulos, T.P.; Vavourakis, E.; Tsochatzis, E.; Pangalis, G.A.; Papatheodoridis, G.; Stamoulakatou, A.; Swinkels, D.W.; Papanikolaou, G.; et al. A Novel Immunological Assay for Hepcidin Quantification in Human Serum. PLoS ONE 2009, 4, e4581. [Google Scholar] [CrossRef]
- Seymour, J.F.; Talpaz, M.; Hagemeister, F.B.; Cabanillas, F.; Kurzrock, R. Clinical Correlates of Elevated Serum Levels of Interleukin-6 in Patients with Untreated Hodgkin’s Disease. Am. J. Med. 1997, 102, 21–28. [Google Scholar] [CrossRef]
- Casasnovas, R.O.; Mounier, N.; Brice, P.; Divine, M.; Morschhauser, F.; Gabarre, J.; Blay, J.Y.; Voillat, L.; Lederlin, P.; Stamatoullas, A.; et al. Plasma Cytokine and Soluble Receptor Signature Predicts Outcome of Patients with Classical Hodgkin’s Lymphoma: A Study from the Groupe d’Etude Des Lymphomes de l’Adulte. J. Clin. Oncol. 2007, 25, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Hutchings, M.; Ramadan, S. Clinical Presentation and Staging of Hodgkin Lymphoma. Semin. Hematol. 2016, 53, 148–154. [Google Scholar] [CrossRef]
- Carbone, P.P.; Kaplan, H.S.; Musshoff, K.; Smithers, D.W.; Tubiana, M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971, 31, 1860–1861. [Google Scholar]
- Bernard, L.; Ferrière, F.; Casassus, P.; Malas, F.; Lévêque, S.; Guillevin, L.; Lortholary, O. Procalcitonin as an Early Marker of Bacterial Infection in Severely Neutropenic Febrile Adults. Clin. Infect. Dis. 1998, 27, 914–915. [Google Scholar] [CrossRef]
- Gendrel, D.; Bohuon, C. Procalcitonin as a Marker of Bacterial Infection. Pediatr. Infect. Dis. J. 2000, 19, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum Procalcitonin and C-Reactive Protein Levels as Markers of Bacterial Infection: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef]
- Henriquez, K.M.; Hayney, M.S.; Rakel, D.P.; Barrett, B. Procalcitonin Levels in Acute Respiratory Infection. Viral Immunol. 2016, 29, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Korppi, M.; Remes, S. Serum Procalcitonin in Pneumococcal Pneumonia in Children. Eur. Respir. J. 2001, 17, 623–627. [Google Scholar] [CrossRef]
- Schuetz, P.; Christ-Crain, M.; Thomann, R.; Falconnier, C.; Wolbers, M.; Widmer, I.; Neidert, S.; Fricker, T.; Blum, C.; Schild, U.; et al. Effect of Procalcitonin-Based Guidelines vs Standard Guidelines on Antibiotic Use in Lower Respiratory Tract Infections: The ProHOSP Randomized Controlled Trial. JAMA J. Am. Med. Assoc. 2009, 302, 1059–1066. [Google Scholar] [CrossRef]
- Bröckelmann, P.J.; Angelopoulou, M.K.; Vassilakopoulos, T.P. Prognostic Factors in Hodgkin Lymphoma. Semin. Hematol. 2016, 53, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Ek, T.; Pinkava, M.; Abrahamsson, J. Ara-C Fever and Infections after High-Dose Ara-C Treatment in Pediatric Lymphoid Malignancies. J. Pediatric Hematol./Oncol. 2005, 27, 364–369. [Google Scholar] [CrossRef]
- Ek, T.; Jarfelt, M.; Mellander, L.; Abrahamsson, J. Proinflammatory Cytokines Mediate the Systemic Inflammatory Response Associated with High-Dose Cytarabine Treatment in Children. Med. Pediatr. Oncol. 2001, 37, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Oberhoffer, M.; Stonans, I.; Russwurm, S.; Stonane, E.; Vogelsang, H.; Junker, U.; Jäger, L.; Reinhart, K. Procalcitonin Expression in Human Peripheral Blood Mononuclear Cells and Its Modulation by Lipopolysaccharides and Sepsis-Related Cytokines in Vitro. J. Lab. Clin. Med. 1999, 134, 49–55. [Google Scholar] [CrossRef]
| Variable | All Patients (n = 137) |
|---|---|
| Age, years (median, IQR) | 36 (15–88) |
| <45 years | 89/137 (65%) |
| Gender | |
| Male | 69/137 (50.4%) |
| Histology | |
| Nodular sclerosis (NS) | 96/137 (70%) |
| Mixed cellularity (MC) | 30/137 (22%) |
| Lymphocyte rich (LR) | 3/137 (2%) |
| Lymphocyte predominance (LP) | 1/137 (1%) |
| Unclassified/Not otherwise specified (NOS) | 7/137 (5%) |
| B symptoms | |
| Yes | 55/137 (40.1%) |
| Ann Arbor stage | |
| I | 15/137 (11%) |
| II | 62/137 (45%) |
| III | 30/137 (22%) |
| IV | 30/137 (22%) |
| Early stage (IA/IIA) | 58/137 (42%) |
| Advanced stage (IB/IIB/III/IV) | 79/137 (58%) |
| Anemia | |
| Yes | 75/137 (55%) |
| White blood cells (WBC) | |
| ≥15 × 109/L | 20/117 (15%) |
| Lymphocytes | |
| <0.6 × 109/L or <8% | 16/136 (12%) |
| Platelet count (median, IQR) | 313 (31–705) |
| ≥400 × 109/L | 35/137 (26%) |
| ESR | |
| ≥50 mm/h | 77/130 (59%) |
| Serum albumin | |
| <4 g/dL | 104/137 (76%) |
| LDH levels | |
| Elevated | 44/137 (32%) |
| β2-microglobulin | |
| Elevated (>2.4 mg/L) | 53/128 (41%) |
| CRP (median, IQR) | 38.1 (2.97–328.0) |
| ≥5 mg/L | 116/137 (85%) |
| Serum Ferritin, ng/mL (median, IQR n = 129) | 154.1 (7–6709) |
| Haptoglobin, mg/dL (median, IQR n = 123) | 298 (66–774) |
| α2-globulin, g/L (median, IQR n = 135) | 0.96 (0.59–1.51) |
| γ-globulin, g/L (median, IQR n = 135) | 1.47 (0.69–3.75) |
| PCT < 0.10 ng/mL | PCT ≥ 0.10 ng/mL * | p | |
|---|---|---|---|
| Total, n (%) | 96 | 41 * | |
| Age, years (median) | 33.5 | 39 | 0.37 |
| Gender (male (#, %)) | 42/96 (44%) | 27/41 (66%) | 0.018 |
| Ann Arbor stage (advanced, IB/IIB/III/IV (#, %)) | 45/96 (47%) | 34/41 (83%) | <0.001 |
| B symptoms (yes (#, %)) | 25/96 (26%) | 30/41 (73%) | <0.001 |
| Histology (NS (#, %)) | 66/94 (70%) | 30/39 (76%) | 0.74 |
| Anemia, g/dL (#, %) | 42/96 (44%) | 33/41 (81%) | <0.001 |
| WBC, ×109/L (≥15 × 109 (#, %)) | 9/96 (9%) | 11/41 (27%) | 0.008 |
| Lymphocytes, × 109/L (<0.6 × 109/L or <8%, (#, %)) | 7/96 (7%) | 9/41 (22%) | 0.014 |
| CRP (median, range) | 25.75 (2.97–203.0) | 92.50 (3.34,328.0) | 0.001 a |
| IQR | 7.96–57.80 | 50.25–130.5 | |
| CRP < 5 mg/L (%) | 20/96 (20.8%) | 1/41 (2.4%) | 0.006 b |
| CRP ≥ 5 mg/L (%) | 76/96 (79.2%) | 40/41 (97.6%) | |
| Highest CRP (mg/L) | 203 | 328 | |
| ESR, mm/h (≥50 mm/h, (#, %)) | 45/91 (50%) | 32/39 (82%) | 0.001 |
| Albumin, g/dl (<4 g/dL (#, %)) | 67/96 (70%) | 37/41 (90%) | 0.01 |
| β2-microglobulin, mg/l (>2.4 mg/L, (#, %)) | 29/88 (33%) | 24/40 (60%) | 0.004 |
| LDH (elevated (#, %)) | 31/96 (32%) | 13/41 (32%) | 0.95 |
| Platelet count, x109/L (median, range) | 297.5 (96–662) | 342.0 (31–705) | 0.059 |
| Serum Ferritin, ng/mL (median, range) | 134.9 (7–1842) | 361.4 (15.3–6709) | <0.001 |
| Haptoglobin, mg/dL (median, range) | 267.5 (66–617) | 431.0 (119–774) | 0.001 |
| α2- globulin, g/L (median, range) | 0.920 (0.60–1.50) | 1.125 (0.59–1.51) | 0.006 |
| γ- globulin, g/L (median, range) | 1.420 (0.69–375) | 1.595 (0.79–3.47) | 0.057 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piperidou, A.; Zografos, E.; Vassilakopoulos, T.J.; Angelopoulou, M.K.; Hillas, G.; Pappis, V.; Boutsikas, G.; Dimou, M.; Petevi, K.; Kanellopoulos, A.; et al. Serum Procalcitonin Levels in Newly Diagnosed Hodgkin Lymphoma: Correlation with Other Inflammatory Biomarkers. Medicina 2022, 58, 1331. https://doi.org/10.3390/medicina58101331
Piperidou A, Zografos E, Vassilakopoulos TJ, Angelopoulou MK, Hillas G, Pappis V, Boutsikas G, Dimou M, Petevi K, Kanellopoulos A, et al. Serum Procalcitonin Levels in Newly Diagnosed Hodgkin Lymphoma: Correlation with Other Inflammatory Biomarkers. Medicina. 2022; 58(10):1331. https://doi.org/10.3390/medicina58101331
Chicago/Turabian StylePiperidou, Alexia, Eleftherios Zografos, Theodoros J. Vassilakopoulos, Maria K. Angelopoulou, Georgios Hillas, Vassiliki Pappis, George Boutsikas, Maria Dimou, Kyriaki Petevi, Alexandros Kanellopoulos, and et al. 2022. "Serum Procalcitonin Levels in Newly Diagnosed Hodgkin Lymphoma: Correlation with Other Inflammatory Biomarkers" Medicina 58, no. 10: 1331. https://doi.org/10.3390/medicina58101331







