Secondary Hemophagocytic Syndrome in a Patient with Plasma Cell Myeloma and CNS Involvement Treated with Lenalidomide
Abstract
:1. Introduction
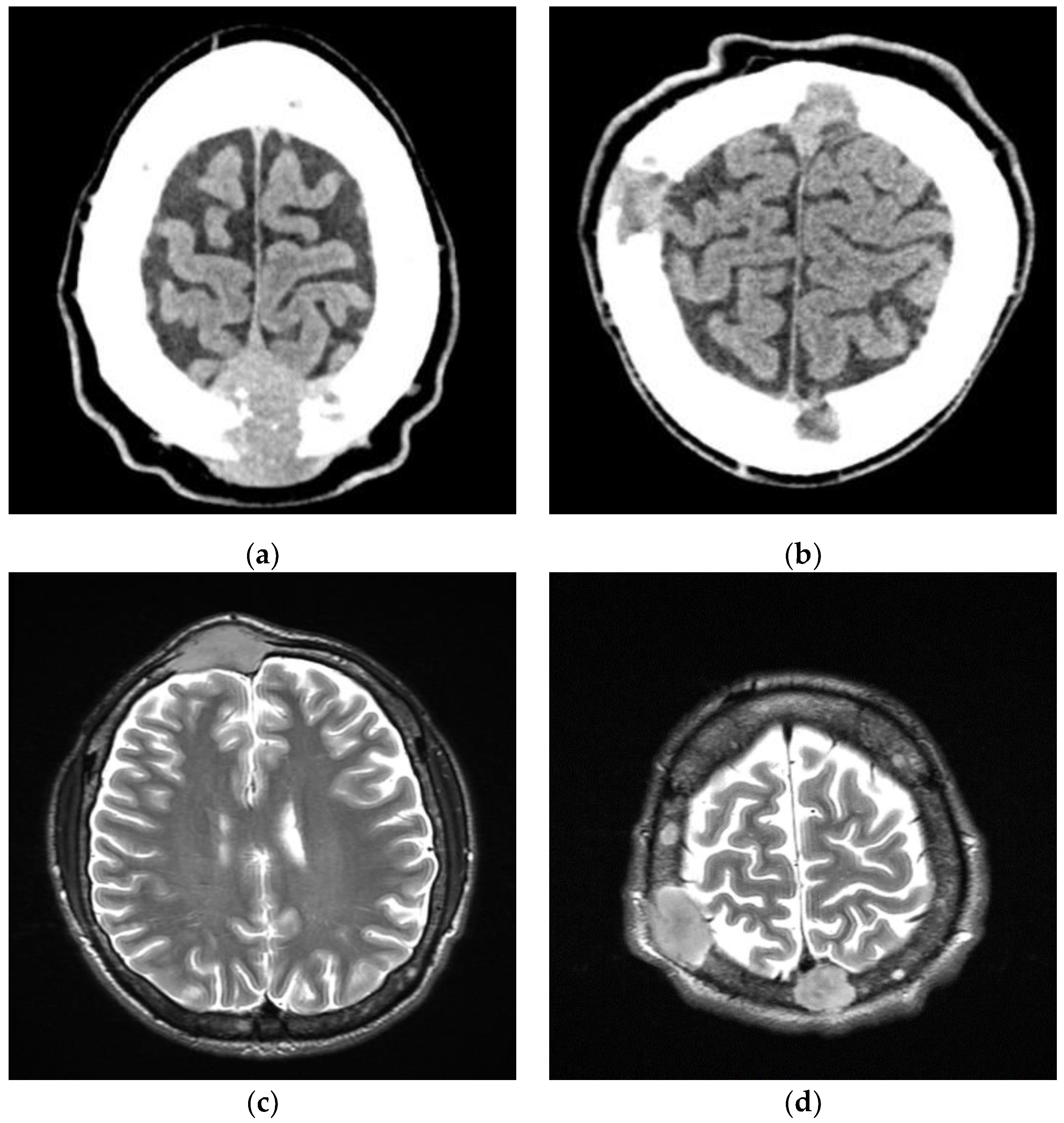
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Katzmann, J.A.; Dispenzieri, A.; Kyle, R.A.; Snyder, M.R.; Plevak, M.F.; Larson, D.R.; Abraham, R.S.; Lust, J.A.; Melton, L.J., 3rd; Rajkumar, S.V. Elimination of the Need for Urine Studies in the Screening Algorithm for Monoclonal Gammopathies by Using Serum Immunofixation and Free Light Chain Assays. Mayo Clin. Proc. 2006, 81, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Fassas, A.B.T.; Muwalla, F.; Berryman, T.; Benramdane, R.; Joseph, L.; Anaissie, E.; Sethi, R.; Desikan, R.; Siegel, D.; Badros, A.; et al. Myeloma of the central nervous system: Association with high-risk chromosomal abnormalities, plasmablastic morphology and extramedullary manifestations: Myeloma of the Central Nervous System. Br. J. Haematol. 2002, 117, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Filipovich, A.; McClain, K.; Grom, A. Histiocytic Disorders: Recent Insights into Pathophysiology and Practical Guidelines. Biol. Blood Marrow Transplant. 2010, 16, S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Horne, A.; Aricó, M.; R Egeler, M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Griffin, G.; Shenoi, S.; Hughes, G.C. Hemophagocytic lymphohistiocytosis: An update on pathogenesis, diagnosis, and therapy. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101515. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brito-Zerón, P.; López-Guillermo, A.; Khamashta, M.A.; Bosch, X. Adult haemophagocytic syndrome. Lancet 2014, 383, 1503–1516. [Google Scholar] [CrossRef]
- Thomas, W.; van’t Veer, M.; Besser, M. Haemophagocytic lymphohistiocytosis: An elusive syndrome. Clin. Med. 2016, 16, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Zeh, D.; Janzen, V.; Mügge, L.-O.; Wolf, D.; Fingerhut, L.; Hahn-Ast, C.; Maurer, O.; Brossart, P.; von Lilienfeld-Toal, M. Treatment with lenalidomide induces immunoactivating and counter-regulatory immunosuppressive changes in myeloma patients. Clin. Exp. Immunol. 2014, 177, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Lioznov, M.; El-Cheikh, J., Jr.; Hoffmann, F.; Hildebrandt, Y.; Ayuk, F.; Wolschke, C.; Atanackovic, D.; Schilling, G.; Badbaran, A.; Bacher, U.; et al. Lenalidomide as salvage therapy after allo-SCT for multiple myeloma is effective and leads to an increase of activated NK (NKp44+) and T (HLA-DR+) cells. Bone Marrow Transplant. 2010, 45, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kneppers, E.; van der Holt, B.; Kersten, M.J.; Kersten, M.; Zweegman, S.; Meijer, E.; Huls, G.; Cornelissen, j.; Janssen, J.J.; Huisman, C.; et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: Results of the HOVON 76 Trial. Blood 2011, 118, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Runge, E.; Kou, C.-T.J.; Rendo, M.; Lynch, D.; Fenderson, J. Lenalidomide-Associated Hemophagocytic Lymphohistiocytosis with Plasma Cell Phagocytosis. Cureus 2021, 13, e14409. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.; Wooten, M.; Thompson Heffner, L.; Waller, E. Daratumumab-associated hemophagocytic lymphohistiocytosis. Ann. Hematol. 2020, 99, 181–182. [Google Scholar] [CrossRef] [PubMed]
- La Rosée, P.; Horne, A.; Hines, M.; van Bahr Greenwood, T.; Machowicz, R.; Berliner, N.; Birndt, S.; Gil-Herrera, J.; Girschikofsky, M.; Jordan, M.B.; et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood 2019, 133, 2465–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milczarek, S.; Kulig, P.; Baumert, B.; Łanocha, A.; Sommerfeld, K.; Borowiecka, E.; Osękowska, B.; Paczkowska, E.; Zdziarska, B.; Machaliński, B. Secondary Hemophagocytic Syndrome in a Patient with Plasma Cell Myeloma and CNS Involvement Treated with Lenalidomide. Medicina 2022, 58, 1350. https://doi.org/10.3390/medicina58101350
Milczarek S, Kulig P, Baumert B, Łanocha A, Sommerfeld K, Borowiecka E, Osękowska B, Paczkowska E, Zdziarska B, Machaliński B. Secondary Hemophagocytic Syndrome in a Patient with Plasma Cell Myeloma and CNS Involvement Treated with Lenalidomide. Medicina. 2022; 58(10):1350. https://doi.org/10.3390/medicina58101350
Chicago/Turabian StyleMilczarek, Sławomir, Piotr Kulig, Bartłomiej Baumert, Aleksandra Łanocha, Krzysztof Sommerfeld, Ewa Borowiecka, Bogumiła Osękowska, Edyta Paczkowska, Barbara Zdziarska, and Bogusław Machaliński. 2022. "Secondary Hemophagocytic Syndrome in a Patient with Plasma Cell Myeloma and CNS Involvement Treated with Lenalidomide" Medicina 58, no. 10: 1350. https://doi.org/10.3390/medicina58101350




