Treatment of Complex Two-Vessel Coronary Heart Disease with Single Left Internal Mammary Artery as T-Graft with Itself—A Retrospective Double Center Analysis of Short-Term Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Applied Criteria for LIMA-LIMA T-Graft
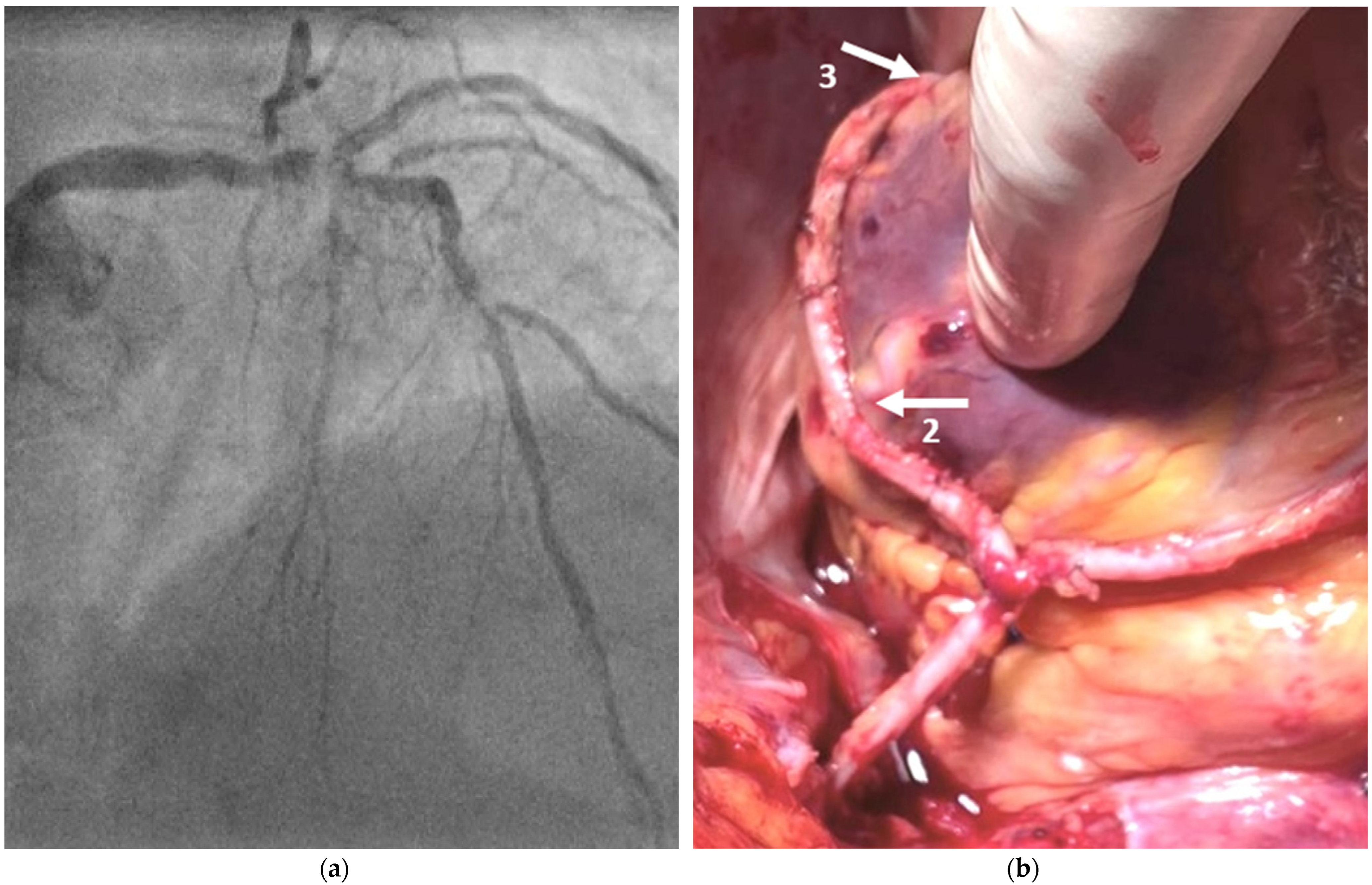
2.2. Surgical Technique
2.3. Survey Objectives
2.4. Statistical Analysis
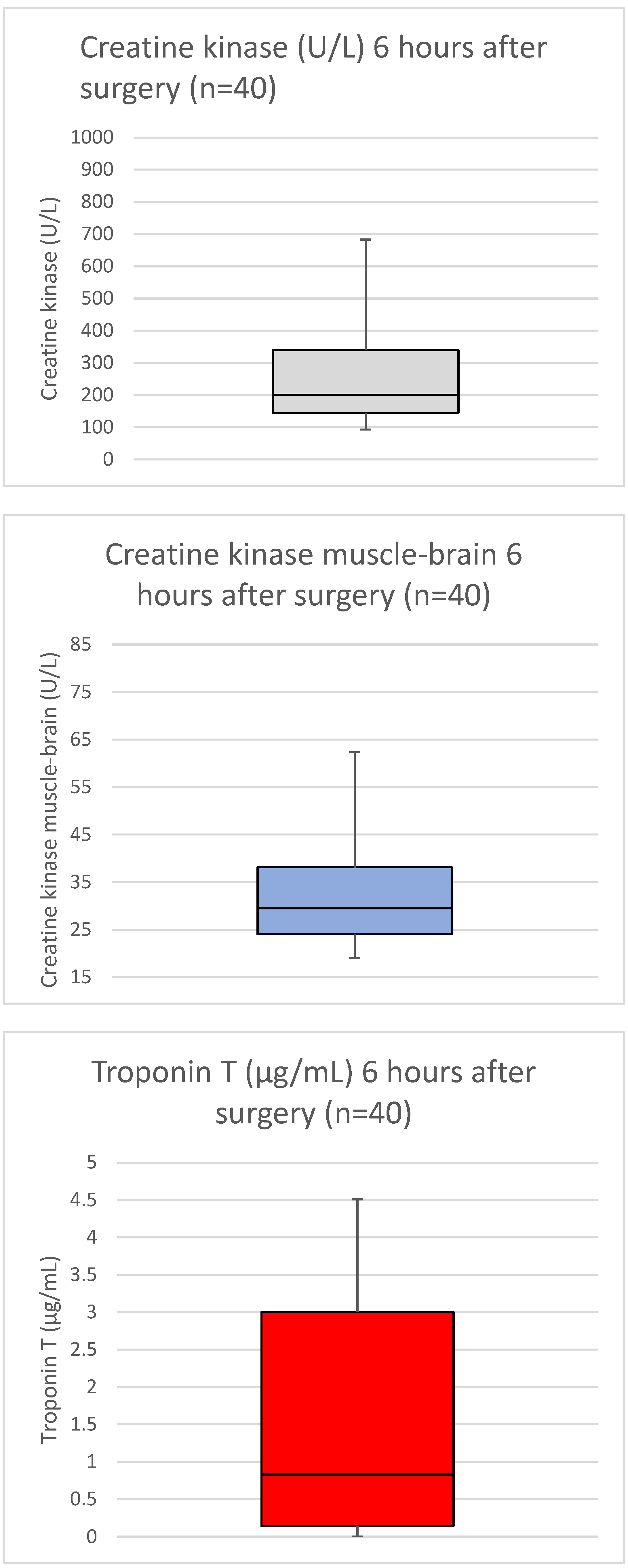
3. Results
4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sousa-Uva, M.; Neumann, F.J.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. J. Cardiothorac. Surg. 2019, 55, 4–90. [Google Scholar] [CrossRef] [PubMed]
- Sears, E.D.; Wu, L.; Waljee, J.F.; Momoh, A.O.; Zhong, L.; Chung, K.C. The Impact of Deep Sternal Wound Infection on Mortality and Resource Utilization: A Population-based Study. World J. Surg. 2016, 40, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Bibo, L.; Pierre, M.; Bayfield, N.; Raichel, L.; Merry, C.; Larbalestier, R. Deep sternal wound infections after cardiac surger: A new Australian tertiary centre experience. Heart Lung Circ. 2020, 29, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Borger, M.A.; Rao, V.; Weisel, R.D.; Ivanov, J.; Cohen, G.; Scully, H.E.; David, T.E. Deep sternal wound infection: Risk factors and outcomes. Ann. Thorac. Surg. 1998, 65, 1050–1056. [Google Scholar] [CrossRef]
- Zenati, M.A.; Bhatt, D.L.; Bakaeen, F.G.; Stock, E.M.; Biswas, K.; Gaziano, J.M.; Kelly, R.F.; Tseng, E.E.; Bitondo, J.; Quin, J.A.; et al. Randomized Trial of Endoscopic or Open Vein-Graft Harvesting for Coronary-Artery Bypass. N. Engl. J. Med. 2019, 380, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Fouquet, O.; Tariel, F.; Desulauze, P.; Mével, G. Does a skeletonized internal thoracic artery give fewer postoperative complications than a pedicled artery for patients undergoing coronary artery bypass grafting? Interact. CardioVasc. Thorac. Surg. 2015, 20, 663–668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fowler, V.G., Jr.; O’Brien, S.M.; Muhlbaier, L.H.; Corey, G.R.; Ferguson, T.B.; Peterson, E.D. Clinical predictors of major infections after cardiac surgery. Circulation 2005, 112 (Suppl. S9), I358–I365. [Google Scholar] [CrossRef] [PubMed]
- Rustenbach, C.J.; Djordjevic, I.; Gerfer, S.; Ivanov, B.; Gaisendrees, C.; Eghbalzadeh, K.; Wahlers, T. Multiple Grafting with Single Left Internal Mammary Artery as T-Graft with Itself. Thorac. Cardiovasc. Surg. 2021; ahead of print. [Google Scholar]
- Ji, Q.; Xia, L.; Shi, Y.; Ma, R.; Shen, J.; Lai, H.; Ding, W.; Wang, C. Sequential Grafting of in Situ Skeletonized Left Internal Mammary Artery to the Left Coronary System. Int. Heart J. 2018, 59, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Akyea, R.K.; Leonardi-Bee, J.; Asselbergs, F.W.; Patel, R.S.; Durrington, P.; Wierzbicki, A.S.; Ibiwoye, O.H.; Kai, J.; Qureshi, N.; Weng, S.F. Predicting major adverse cardiovascular events for secondary prevention: Protocol for a systematic review and meta-analysis of risk prediction models. BMJ 2020, 10, e034564. [Google Scholar] [CrossRef] [PubMed]
- SYNTAX Score Calculator. Available online: www.syntaxscore.com/calculator/start.htm (accessed on 1 March 2019).
- Sianos, G.; Morel, M.A.; Kappetein, L.A.P. The SYNTAX Score: An angiographic toolgrading to complexity of coronary artery disease. EuroIntervention 2005, 51, 219–227. [Google Scholar]
- Schwann, T.A.; Tatoulis, J.; Puskas, J.; Bonnell, M.; Taggart, D.; Kurlansky, P.; Jacobs, J.P.; Thourani, V.H.; O’Brien, S.; Wallace, A.; et al. Worldwide Trends in Multi-arterial Coronary Artery Bypass Grafting Surgery 2004–2014: A Tale of 2 Continents. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, J.; Heeren, A.; Szecel, D.; Meuris, B.; Jacobs, S.; Verbrugghe, P.; Oosterlinck, W. Skeletonisation contributing to a reduction of sternal wound complications: A retrospective study in OPCAB patients. J. Cardiothorac. Surg. 2019, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Taggart, D.P.; Benedetto, U.; Gerry, S.; Altman, D.G.; Gray, A.M.; Lees, B.; Gaudino, M.; Zamvar, V.; Bochenek, A.; Buxton, B.; et al. Arterial Revascularization Trial Investigators. Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N. Engl. J. Med. 2019, 830, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Samadashvili, Z.; Sundt, T.M., 3rd; Wechsler, A.; Chikwe, J.; Adams, D.H.; Smith, C.R.; Jordan, D.; Girardi, L.; Lahey, S.J.; Gold, J.P.; et al. Multiple Versus Single Arterial Coronary Bypass Graft Surgery for Multivessel Disease. J. Am. Coll. Cardiol. 2019, 74, 1275–1285. [Google Scholar] [CrossRef] [PubMed]

| Parameter | n = 40 |
|---|---|
| Age—years | 71.9 ± 7.1 |
| Male sex—n (%) | 27 (67.5) |
| Body-mass index | 30.1 ± 2.3 |
| Current smoker—n (%) | 11 (27.5) |
| Ex-smoker—n (%) | 24 (60.0) |
| Co-morbidities | |
| Hyperlipidemia—n (%) | 40 (100) |
| Hypertension—n (%) | 39 (97.5) |
| Pre-existent Stroke—n (%) | 3 (7.5) |
| Chronic lung disease—n (%) | 8 (44.5) |
| Immunosuppressive therapy—n (%) | 3 (7.5) |
| Peripheral vascular disease—n (%) | 19 (47.5) |
| Cerebrovascular disease—n (%) | 6 (15.0) |
| Previous percutaneous coronary intervention—n (%) | 18 (45.0) |
| Previous coronary artery bypass surgery—n (%) | 7 (17.5) |
| Previous valve surgery—n (%) | 2 (5.0) |
| Diabetes mellitus—n (%) | 27 (67.5) |
| With HbA1c > 8.5—n (%) | 7 (17.5) |
| Previous myocardial infarction—n (%) | 6 (33.3) |
| Atrial fibrillation—n (%) | 11 (27.5) |
| Dialysis—n (%) | 0 (0) |
| Renal failure, without dialysis—n (%) | 4 (10.0) |
| NYHA class IV—n (%) | 3 (7.5) |
| Ejection fraction | 46.1 ± 8.5 |
| Cardiogenic shock—n (%) | 0 (0) |
| Syntax – Score | 32.1 ± 7.4 |
| LIMA-LIMA T-graft criteria | |
| Varicosis—n (%) | 21 (52.5) |
| Vein stripping—n (%) | 15 (37.5) |
| Allen’s test positive—n (%) | 26 (65.0) |
| Fowler score | 20.1 ± 3.0 |
| Parameter | n = 40 |
|---|---|
| Urgency of procedures | |
| Elective—n (%) | 23 (57.5) |
| Urgent—n (%) | 10 (25.0) |
| Emergent—n (%) | 7 (17.5) |
| Operative data | |
| Number of distal anastomoses | 2.6 ± 0.5 |
| Flow LIMA-LAD (mL/min) | 59.31 ± 11.04 |
| PI LIMA-LAD | 1.21 ± 0.18 |
| Flow T-graft (mL/min) | 41.31 ± 3.81 |
| PI T-graft | 1.39 ± 0.47 |
| Incision to closure time (min) | 121.5 (106.7; 150) |
| Parameter | n = 40 |
|---|---|
| Postoperative complications | |
| Deep sternal wound infection | 0 (0%) |
| Wound healing disorder | 1 (2.5%) |
| Re-thoracotomy | 0 (0%) |
| Major adverse cardiac events | 0 (0%) |
| Outcomes | |
| ICU stay (days) | 1.0 ± 0.4 |
| Total hospital stay (days) | 6.6 (6.0; 7.2) |
| Discharged from hospital | 40 (100%) |
| Intra-hospital mortality | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rustenbach, C.J.; Djordjevic, I.; Eghbalzadeh, K.; Baumbach, H.; Wendt, S.; Radwan, M.; Marinos, S.L.; Mustafi, M.; Lescan, M.; Berger, R.; et al. Treatment of Complex Two-Vessel Coronary Heart Disease with Single Left Internal Mammary Artery as T-Graft with Itself—A Retrospective Double Center Analysis of Short-Term Outcomes. Medicina 2022, 58, 1415. https://doi.org/10.3390/medicina58101415
Rustenbach CJ, Djordjevic I, Eghbalzadeh K, Baumbach H, Wendt S, Radwan M, Marinos SL, Mustafi M, Lescan M, Berger R, et al. Treatment of Complex Two-Vessel Coronary Heart Disease with Single Left Internal Mammary Artery as T-Graft with Itself—A Retrospective Double Center Analysis of Short-Term Outcomes. Medicina. 2022; 58(10):1415. https://doi.org/10.3390/medicina58101415
Chicago/Turabian StyleRustenbach, Christian Jörg, Ilija Djordjevic, Kaveh Eghbalzadeh, Hardy Baumbach, Stefanie Wendt, Medhat Radwan, Spiro Lukas Marinos, Migdat Mustafi, Mario Lescan, Rafal Berger, and et al. 2022. "Treatment of Complex Two-Vessel Coronary Heart Disease with Single Left Internal Mammary Artery as T-Graft with Itself—A Retrospective Double Center Analysis of Short-Term Outcomes" Medicina 58, no. 10: 1415. https://doi.org/10.3390/medicina58101415
APA StyleRustenbach, C. J., Djordjevic, I., Eghbalzadeh, K., Baumbach, H., Wendt, S., Radwan, M., Marinos, S. L., Mustafi, M., Lescan, M., Berger, R., Salewski, C., Sandoval Boburg, R., Steger, V., Nemeth, A., Reichert, S., Wahlers, T., & Schlensak, C. (2022). Treatment of Complex Two-Vessel Coronary Heart Disease with Single Left Internal Mammary Artery as T-Graft with Itself—A Retrospective Double Center Analysis of Short-Term Outcomes. Medicina, 58(10), 1415. https://doi.org/10.3390/medicina58101415











