Gait Analysis of Bilateral Knee Osteoarthritis and Its Correlation with Western Ontario and McMaster University Osteoarthritis Index Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Gait Analyser Models and Precautions
2.3. Gait Data Collection
2.4. Weight-Bearing Balance and Walking Stability Assessment
2.5. Scoring Scale
2.6. Statistical Analysis
3. Results
3.1. Gait Analysis
3.2. Weight-Bearing Balance and Walking Stability Assessment
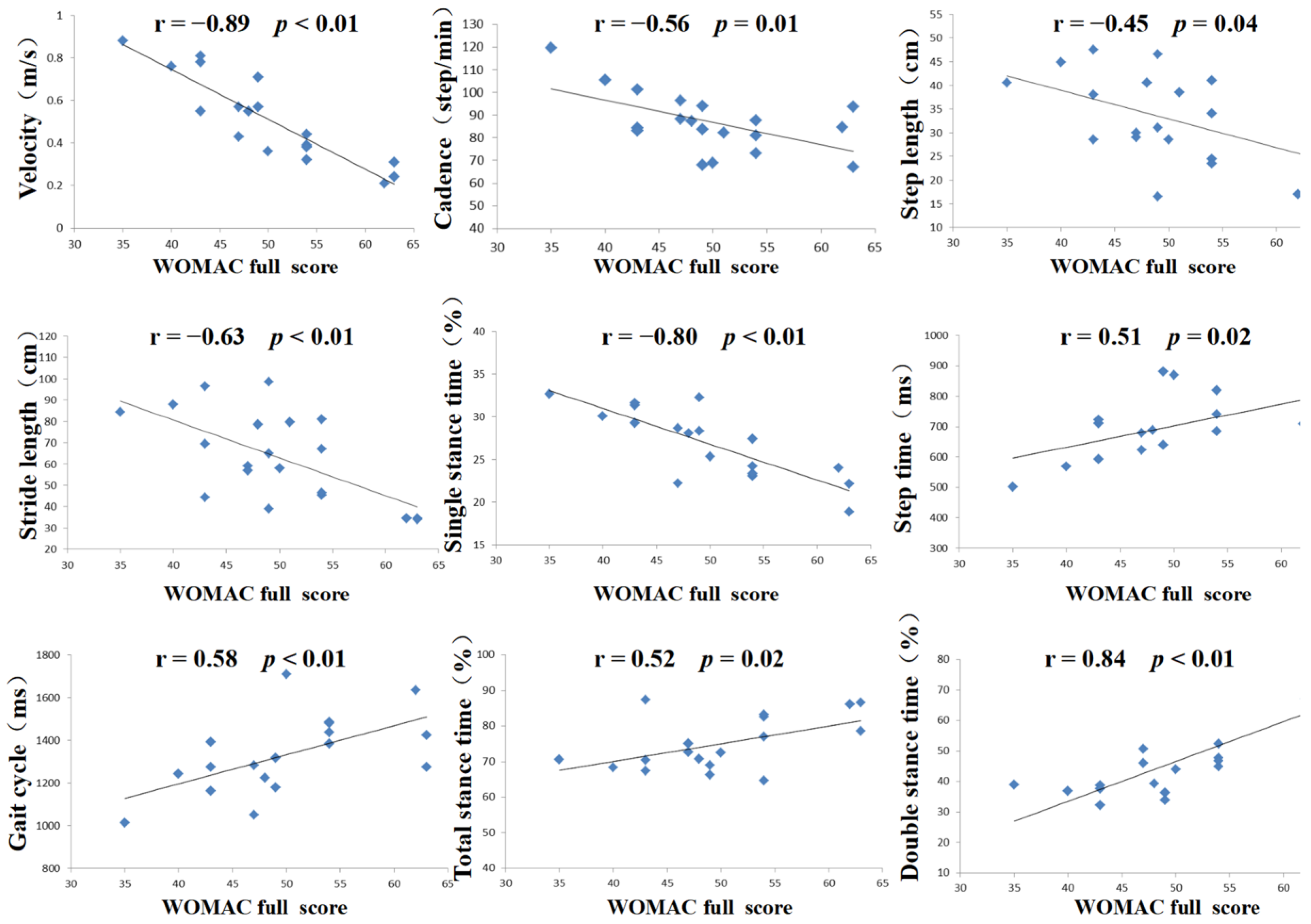
3.3. WOMAC Score and Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bączkowicz, D.; Skiba, G.; Czerner, M.; Majorczyk, E. Gait and functional status analysis before and after total knee arthroplasty. Knee 2018, 25, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Bonnefoy-Mazure, A.; Favre, T.; Praplan, G.; Armand, S.; Sagawa Junior, Y.; Hannouche, D.; Turcot, K.; Lübbeke, A.; Miozzari, H.H. Associations between gait analysis parameters and patient satisfaction one year following primary total knee arthroplasty. Gait Posture 2020, 80, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, J.; Casaña, J.; Ezzatvar, Y.; Jakobsen, M.D.; Sundstrup, E.; Andersen, L.L. High-intensity preoperative training improves physical and functional recovery in the early post-operative periods after total knee arthroplasty: A randomized controlled trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.L.; Truong, L.K.; Dhiman, K.; Beck, C. Osteoarthritis year in review 2020: Rehabilitation and outcomes. Osteoarthr. Cartilage 2021, 29, 190–207. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, K.M.; Han, H.; Koo, S.; Kang, S.B.; Chang, C.B. Relationship between radiographic measurements and knee adduction moment using 3D gait analysis. Gait Posture 2021, 90, 179–184. [Google Scholar] [CrossRef]
- Wada, S.; Murakami, H.; Tajima, G.; Maruyama, M.; Sugawara, A.; Oikawa, S.; Chida, Y.; Doita, M. Analysis of characteristics required for gait evaluation of patients with knee osteoarthritis using a wireless accelerometer. The Knee 2021, 32, 37–45. [Google Scholar] [CrossRef]
- Naili, J.E.; Iversen, M.D.; Esbjörnsson, A.C.; Hedström, M.; Schwartz, M.H.; Häger, C.K.; Broström, E.W. Deficits in functional performance and gait one year after total knee arthroplasty despite improved self-reported function. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3378–3386. [Google Scholar] [CrossRef] [Green Version]
- Low, D.C.; Dixon, S.J. Footscan pressure insoles: Accuracy and reliability of force and pressure measurements in running. Gait Posture 2010, 32, 664–666. [Google Scholar] [CrossRef]
- Sharpe, T.; Malone, A.; French, H.; Kiernan, D.; O’Brien, T. Effect of flip-flops on lower limb kinematics during walking: A cross-sectional study using three-dimensional gait analysis. Ir. J. Med. Sci. 2016, 185, 493–501. [Google Scholar] [CrossRef]
- Zhao, R.; Dong, Z.; Wei, X.; Gu, X.; Han, P.; Wu, H.; Yan, Y.; Huang, L.; Li, H.; Zhang, C.; et al. Inflammatory factors are crucial for the pathogenesis of post-traumatic osteoarthritis confirmed by a novel porcine model: “Idealized” anterior cruciate ligament reconstruction” and gait analysis. Int. Immunopharmacol. 2021, 99, 107905. [Google Scholar] [CrossRef]
- Hughes-Oliver, C.N.; Srinivasan, D.; Schmitt, D.; Queen, R.M. Gender and limb differences in temporal gait parameters and gait variability in ankle osteoarthritis. Gait Posture 2018, 65, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Milner, C.E. Interlimb asymmetry during walking following unilateral total knee arthroplasty. Gait Posture 2008, 28, 69–73. [Google Scholar] [CrossRef]
- Deyle, G.D.; Allen, C.S.; Allison, S.C.; Gill, N.W.; Hando, B.R.; Petersen, E.J.; Dusenberry, D.I.; Rhon, D.I. Physical Therapy versus Glucocorticoid Injection for Osteoarthritis of the Knee. N. Engl. J. Med. 2020, 382, 1420–1429. [Google Scholar] [CrossRef]
- Du, C.; Smith, A.; Avalos, M.; South, S.; Crabtree, K.; Wang, W.; Kwon, Y.H.; Vijayagopal, P.; Juma, S. Blueberries Improve Pain, Gait Performance, and Inflammation in Individuals with Symptomatic Knee Osteoarthritis. Nutrients 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, R.; Esquenazi, A.; Benedetti, M.G.; Desloovere, K. Gait analysis: Clinical facts. Eur. J. Phys. Rehabil. Med. 2016, 52, 560–574. [Google Scholar] [PubMed]
- Klöpfer-Krämer, I.; Brand, A.; Wackerle, H.; Müßig, J.; Kröger, I.; Augat, P. Gait analysis—Available platforms for outcome assessment. Injury 2020, 51 (Suppl. S2), S90–S96. [Google Scholar] [CrossRef]
- Lakes, E.H.; Allen, K.D. Gait analysis methods for rodent models of arthritic disorders: Reviews and recommendations. Osteoarthr. Cartil. 2016, 24, 1837–1849. [Google Scholar] [CrossRef] [Green Version]
- Creaby, M.W.; Bennell, K.L.; Hunt, M.A. Gait differs between unilateral and bilateral knee osteoarthritis. Arch. Phys. Med. Rehabil. 2012, 93, 822–827. [Google Scholar] [CrossRef]
- Lyle, M.A.; Jensen, J.C.; Hunnicutt, J.L.; Brown, J.J.; Chambliss, C.P.; Newsome, M.A.; Xerogeanes, J.W.; Tsai, L.-C. Identification of strength and spatiotemporal gait parameters associated with knee loading during gait in persons after anterior cruciate ligament reconstruction. J. Athl. Train. 2021; preprint. [Google Scholar] [CrossRef]
- Sagawa, Y., Jr.; Armand, S.; Lubbeke, A.; Hoffmeyer, P.; Fritschy, D.; Suva, D.; Turcot, K. Associations between gait and clinical parameters in patients with severe knee osteoarthritis: A multiple correspondence analysis. Clin. Biomech. 2013, 28, 299–305. [Google Scholar] [CrossRef]
- Schrijvers, J.C.; van den Noort, J.C.; van der Esch, M.; Dekker, J.; Harlaar, J. Objective parameters to measure (in) stability of the knee joint during gait: A review of literature. Gait Posture 2019, 70, 235–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, S.M.; Astephen Wilson, J.L.; Rutherford, D.J.; Hubley-Kozey, C.L. Reliability of principal components and discrete parameters of knee angle and moment gait waveforms in individuals with moderate knee osteoarthritis. Gait Posture 2013, 38, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Hong, P.W.; Chen, C.L.; Chou, S.W.; Wu, C.-Y.; Cheng, P.-T.; Tang, F.-T.; Chen, H.-C. Ground reaction force patterns in stroke patients with various degrees of motor recovery determined by plantar dynamic analysis. Chang Gung Med. J. 2007, 30, 62–72. [Google Scholar] [PubMed]
- Viteckova, S.; Kutilek, P.; Svoboda, Z.; Krupicka, R.; Kauler, J.; Szabo, Z. Gait symmetry measures: A review of current and prospective methods. Biomed. Signal Process. Control 2018, 42, 89–100. [Google Scholar] [CrossRef]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and limb dominance in able-bodied gait: A review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Cudkowicz, M.E.; Firtion, R.; Wei, J.Y.; Goldberger, A.L. Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov. Disord. 1998, 13, 428–437. [Google Scholar] [CrossRef]
- Sheridan, P.L.; Solomont, J.; Kowall, N.; Hausdorff, J.M. Influence of executive function on locomotor function: Divided attention increases gait variability in Alzheimer’s disease. J. Am. Geriatr. Soc. 2003, 51, 1633–1637. [Google Scholar] [CrossRef] [Green Version]
- Hausdorff, J.M.; Edelberg, H.K.; Mitchell, S.L.; Goldberger, A.L.; Wei, J.Y. Increased gait unsteadiness in community-dwelling elderly fallers. Arch. Phys. Med. Rehabil. 1997, 78, 278–283. [Google Scholar] [CrossRef]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration patterns of the head and pelvis when walking are associated with risk of falling in community-dwelling older people. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, M446–M452. [Google Scholar] [CrossRef] [Green Version]
- Bascuas, I.; Tejero, M.; Monleón, S.; Boza, R.; Muniesa, J.M.; Belmonte, R. Balance 1 year after TKA: Correlation with clinical variables. Orthopedics 2013, 36, e6–e12. [Google Scholar] [CrossRef]
- Bozbas, T.G.; Sendur, O.F.; Aydemir, A.H. Primary knee osteoarthritis increases the risk of falling. J. Back Musculoskelet. Rehabil. 2017, 30, 785–789. [Google Scholar] [CrossRef] [PubMed]
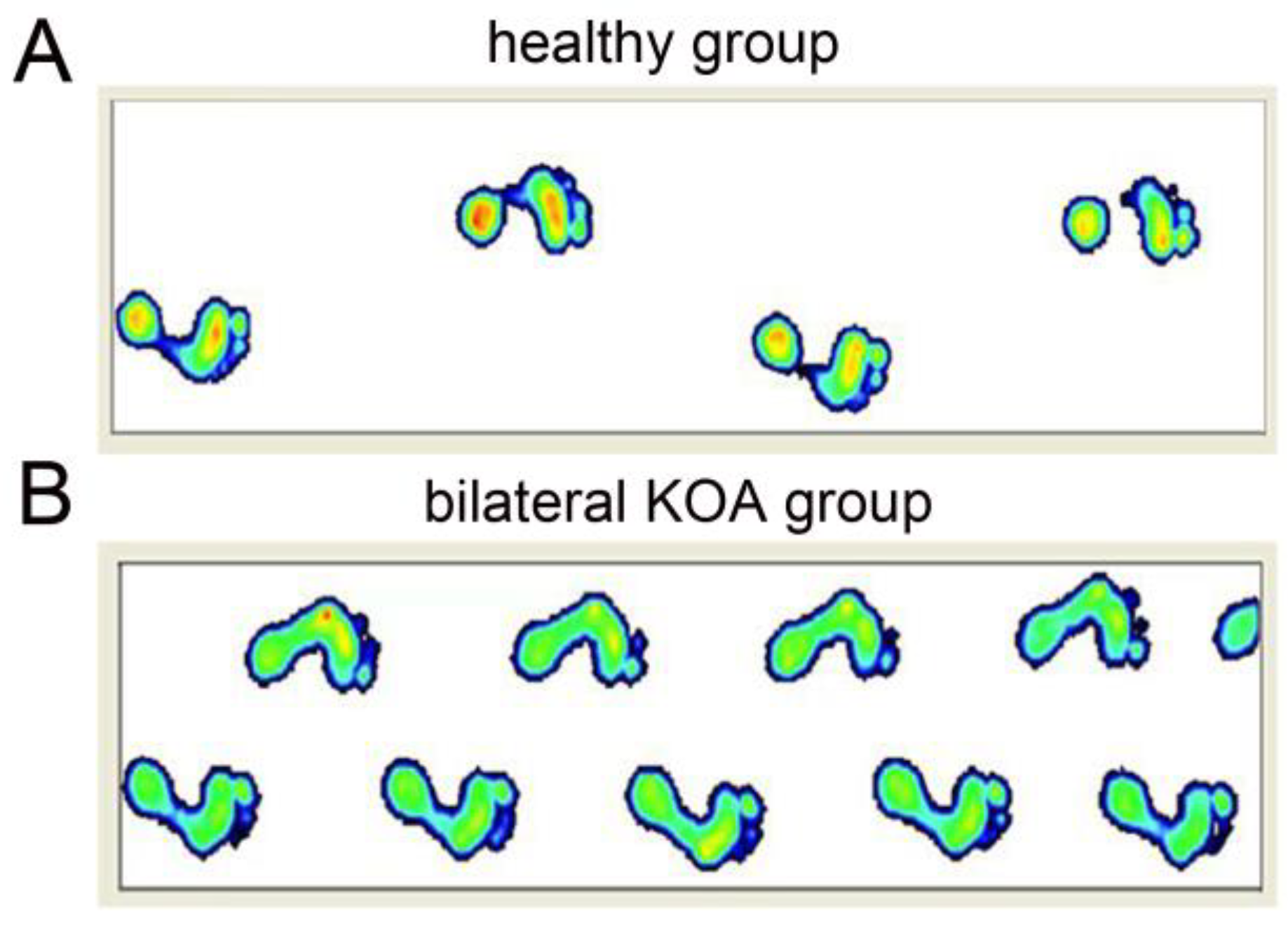
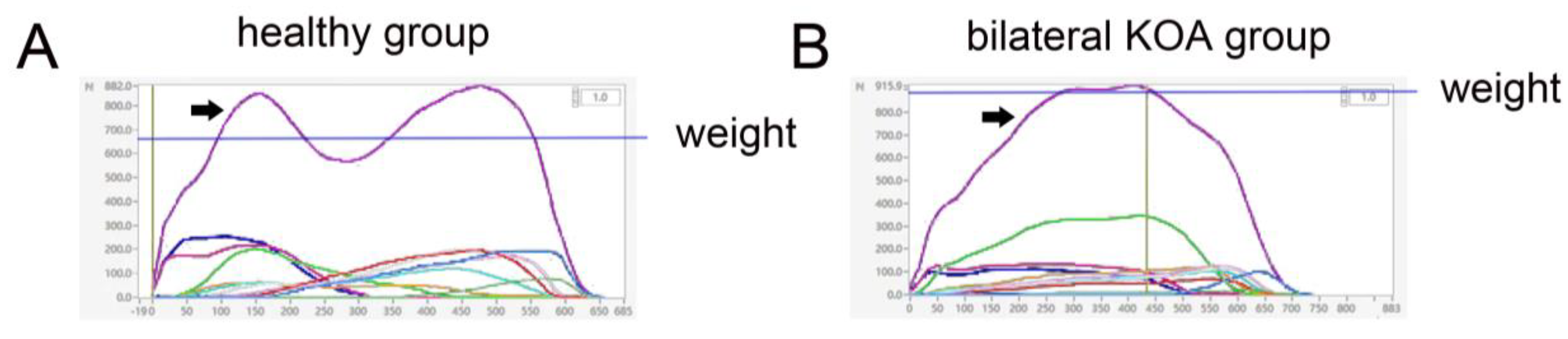
| Parameter | Experimental Group | Control Group | p Value |
|---|---|---|---|
| Gender (Male/Female) | 9/11 | 10/10 | 1 |
| Age (year) | 66.60 ± 6.13 | 65.50 ± 8.37 | 0.65 |
| Height (cm) | 166.50 ± 5.21 | 165.35 ± 7.29 | 0.58 |
| weight (Kg) | 73.15 ± 7.32 | 69.10 ± 8.65 | 0.13 |
| Gait Parameters | Control Group | Experimental Group | 95% CI | p Value |
|---|---|---|---|---|
| Velocity (m/s) | 1.02 ± 0.19 | 0.52 ± 0.19 | (0.38, 0.63) | <0.01 |
| Cadence (step/min) | 109.84 ± 12.99 | 86.87 ± 12.56 | (14.57, 31.36) | <0.01 |
| Step length (cm) | 51.90 ± 4.74 | 32.98 ± 9.65 | (13.92, 23.92) | <0.01 |
| Stride length (cm) | 103.09 ± 8.64 | 62.6 ± 20.58 | (29.73, 50.4) | <0.01 |
| Step time (ms) | 553.34 ± 60.62 | 704.8 ± 99.75 | (−205.7, −97.23) | <0.01 |
| Gait cycle (ms) | 1087.84 ± 117.24 | 1327.95 ± 169.3 | (−335.8, −144.4) | <0.01 |
| Total stance time (%) | 63.62 ± 4.58 | 74.68 ± 6.93 | (−14.92, −7.19) | <0.01 |
| Double-stance time (%) | 31.31 ± 2.43 | 46.31 ± 11.29 | (−20.37, −9.63) | <0.01 |
| Single-stance time (%) | 33.31 ± 1.24 | 26.96 ± 3.78 | (4.50, 8.2) | <0.01 |
| Left knee range of motion (°) | 62.92 ± 4.20 | 38.84 ± 6.74 | (20.40, 27.77) | <0.01 |
| Maximum left knee flexion (°) | 66.26 ± 3.47 | 53.57 ± 4.76 | (9.95, 15.43) | <0.01 |
| Minimum left knee extension (°) | 3.37 ± 1.69 | 14.73 ± 3.88 | (−13.33, −9.39) | <0.01 |
| Right knee range of motion (°) | 62.16 ± 2.86 | 37.51 ± 5.16 | (21.9, 27.39) | <0.01 |
| Maximum right knee flexion (°) | 65.81 ± 2.67 | 53.78 ± 5.15 | (9.34, 14.73) | <0.01 |
| Minimum right knee extension (°) | 3.18 ± 1.48 | 16.27 ± 2.88 | (−14.59, −11.58) | <0.01 |
| Static Standing Bipedal Weight Bearing | Dynamic Walking Bipedal Pressure | |||
|---|---|---|---|---|
| Control Group | Experimental Group | Control Group | Experimental Group | |
| Variance | 0.01 | 0.15 | 0.001 | 0.01 |
| Standard deviation | 0.10 | 0.39 | 0.04 | 0.11 |
| Quartile range | 0.21 | 0.43 | 0.07 | 0.19 |
| Range | 0.30 | 1.62 | 0.11 | 0.38 |
| Step Length | Step Time | |||
|---|---|---|---|---|
| Control Group | Experimental Group | Control Group | Experimental Group | |
| Variance | 0.001 | 0.06 | 0.001 | 0.02 |
| Standard deviation | 0.03 | 0.24 | 0.03 | 0.15 |
| Quartile range | 0.05 | 0.32 | 0.04 | 0.18 |
| Range | 0.13 | 1.22 | 0.11 | 0.55 |
| Pain (Points) | Stiffness (Points) | Daily Living Function (Points) | Full Score (Points) | |
|---|---|---|---|---|
| Experimental group | 10.15 ± 1.67 | 2.80 ± 0.81 | 36.95 ± 5.47 | 49.90 ± 7.22 |
| Pain | Stiffness | Daily Living Function | Full Score | |||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Velocity | −0.66 | <0.01 | −0.63 | <0.01 | −0.88 | <0.01 | −0.89 | <0.01 |
| Cadence | −0.46 | 0.04 | −0.19 | 0.43 | −0.58 | <0.01 | −0.56 | 0.01 |
| Step length | −0.27 | 0.25 | −0.09 | 0.72 | −0.5 | 0.02 | −0.45 | 0.04 |
| Stride length | −0.39 | 0.09 | −0.38 | 0.10 | −0.66 | <0.01 | −0.63 | <0.01 |
| Step time | 0.44 | 0.06 | 0.10 | 0.67 | 0.53 | 0.02 | 0.51 | 0.02 |
| Gait cycle | 0.34 | 0.14 | 0.3 | 0.19 | 0.62 | <0.01 | 0.58 | <0.01 |
| Total stance time | 0.40 | 0.08 | 0.40 | 0.09 | 0.51 | 0.02 | 0.52 | 0.02 |
| Double-stance time | 0.65 | <0.01 | 0.73 | <0.01 | 0.80 | <0.01 | 0.84 | <0.01 |
| Single-stance time | −0.55 | 0.01 | −0.58 | <0.01 | −0.82 | <0.01 | −0.80 | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Hu, S.; Zhao, R.; Zhang, Y.; Huang, L.; Shi, J.; Li, P.; Wei, X. Gait Analysis of Bilateral Knee Osteoarthritis and Its Correlation with Western Ontario and McMaster University Osteoarthritis Index Assessment. Medicina 2022, 58, 1419. https://doi.org/10.3390/medicina58101419
Li H, Hu S, Zhao R, Zhang Y, Huang L, Shi J, Li P, Wei X. Gait Analysis of Bilateral Knee Osteoarthritis and Its Correlation with Western Ontario and McMaster University Osteoarthritis Index Assessment. Medicina. 2022; 58(10):1419. https://doi.org/10.3390/medicina58101419
Chicago/Turabian StyleLi, Haoqian, Shuai Hu, Ruipeng Zhao, Yixuan Zhang, Lingan Huang, Junjun Shi, Pengcui Li, and Xiaochun Wei. 2022. "Gait Analysis of Bilateral Knee Osteoarthritis and Its Correlation with Western Ontario and McMaster University Osteoarthritis Index Assessment" Medicina 58, no. 10: 1419. https://doi.org/10.3390/medicina58101419






