The Predictive Value of Eosinophil Indices for Major Cardiovascular Events in Patients with Acute Decompensated HFrEF
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
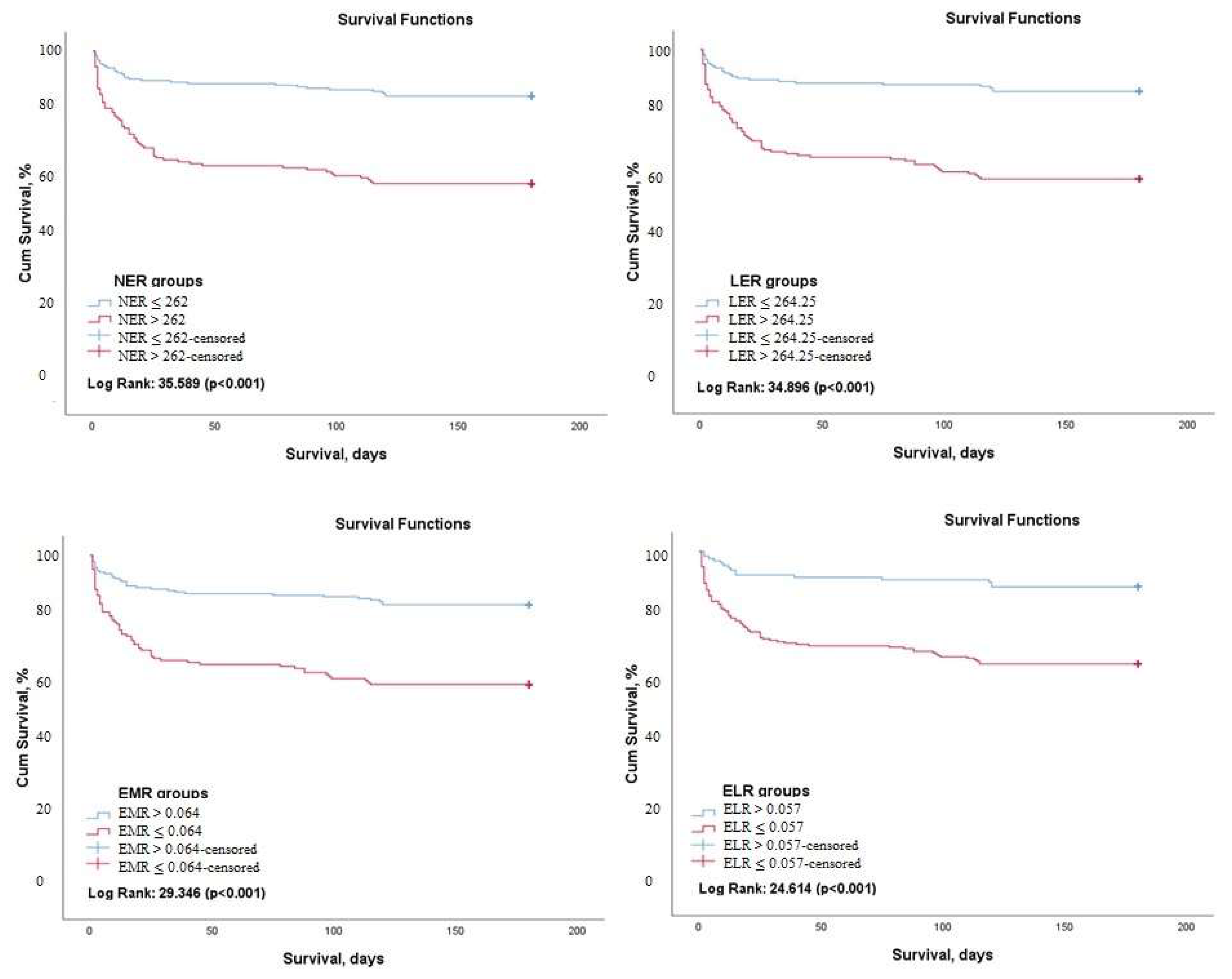
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jhund, P.S.; MacIntyre, K.; Simpson, C.R.; Lewsey, J.D.; Stewart, S.; Redpath, A.; Chalmers, J.W.; Capewell, S.; McMurray, J.J. Long-term trends in first hospitalization for heart failure and subsequent survival between 1986 and 2003: A population study of 5.1 million people. Circulation 2009, 119, 515–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.R.; Taylor, C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.E.; Manuchehry, A.; Chana, A.; Rossi, J.; Schrier, R.W.; Burnett, J.C.; Gheorghiade, M. Prognostic markers in heart failure--congestion, neurohormones, and the cardiorenal syndrome. Acute Card. Care 2007, 9, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Yndestad, A.; Damås, J.K.; Øie, E.; Ueland, T.; Gullestad, L.; Aukrust, P. Role of inflammation in the progression of heart failure. Curr. Cardiol. Rep. 2007, 9, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Yucel, O.; Günes, H.; Kerkütlüoglu, M.; Yılmaz, M.B. C-reactive protein/albumin ratio designates advanced heart failure among outpatients with heart failure. Int. J. Cardiovasc. Acad. 2020, 6, 51. [Google Scholar]
- Curran, F.M.; Bhalraam, U.; Mohan, M.; Singh, J.S.; Anker, S.D.; Dickstein, K.; Doney, A.S.; Filippatos, G.; George, J.; Metra, M. Neutrophil-to-lymphocyte ratio and outcomes in patients with new-onset or worsening heart failure with reduced and preserved ejection fraction. ESC Heart Fail. 2021, 8, 3168–3179. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Frantz, S.; Falcao-Pires, I.; Balligand, J.L.; Bauersachs, J.; Brutsaert, D.; Ciccarelli, M.; Dawson, D.; de Windt, L.J.; Giacca, M.; Hamdani, N. The innate immune system in chronic cardiomyopathy: A European Society of Cardiology (ESC) scientific statement from the Working Group on Myocardial Function of the ESC. Eur. J. Heart Fail. 2018, 20, 445–459. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschöpe, C. Inflammation–cause or consequence of heart failure or both? Curr. Heart FaiL. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, D.L.; Topkara, V.K.; Evans, S.; Barger, P.M. Innate immunity in the adult mammalian heart: For whom the cell tolls. Trans. Am. Clin. Climatol. Assoc. 2010, 121, 34–50. [Google Scholar] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The biological basis for cardiac repair after myocardial infarction: From inflammation to fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Cardioimmunology: The immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Dutka, M.; Bobiński, R.; Ulman-Włodarz, I.; Hajduga, M.; Bujok, J.; Pająk, C.; Ćwiertnia, M. Various aspects of inflammation in heart failure. Heart Fail. Rev. 2020, 25, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Wang, X.; Shen, L.; Yao, K.; Ge, L.; Ma, J.; Zhang, F.; Qian, J.; Ge, J. Association of eosinophil-to-monocyte ratio with 1-month and long-term all-cause mortality in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J. Thorac. Dis. 2018, 10, 5449–5458. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Luo, Y.; Zhang, T.; Huang, C.; Fu, Y.; Zhang, Q.; Zeng, F.; Huang, H.; Zhang, C.; Guo, Z. Eosinophil-to-monocyte ratio is a potential biomarker in the prediction of functional outcome among patients with acute ischemic stroke. BMC Neurosci. 2021, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ren, J.; Yang, N.; Huang, H.; Hu, X.; Sun, F.; Zeng, T.; Zhou, X.; Pan, W.; Hu, J.; et al. Eosinophil-to-Monocyte Ratio is a Potential Predictor of Prognosis in Acute Ischemic Stroke Patients After Intravenous Thrombolysis. Clin. Interv. Aging 2021, 16, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Cikrikcioglu, M.A.; Soysal, P.; Dikerdem, D.; Cakirca, M.; Kazancioglu, R.; Yolbas, S.; Erkal, H.; Hursitoglu, M.; Karakose, T.K.; Kiskac, M.; et al. Absolute blood eosinophil count and 1-year mortality risk following hospitalization with acute heart failure. Eur. J. Emerg. Med. 2012, 19, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, W.; Zhao, L.; Li, Y.; Wang, L.; Mo, F.; Guo, W. Relationship Between the Eosinophil/Monocyte Ratio and Prognosis in Decompensated Heart Failure: A Retrospective Study. J. Inflamm. Res. 2021, 14, 4687–4696. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fukui, M.; Tomiyasu, K.-i.; Akabame, S.; Nakano, K.; Yamasaki, M.; Hasegawa, G.; Oda, Y.; Nakamura, N. Eosinophil count is positively correlated with coronary artery calcification. Hypertens. Res. 2012, 35, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yang, C.; Liu, T.; Deng, Z.; Fang, W.; Zhang, X.; Li, J.; Huang, Q.; Liu, C.; Wang, Y.; et al. Eosinophils improve cardiac function after myocardial infarction. Nat. Commun. 2020, 11, 6396. [Google Scholar] [CrossRef] [PubMed]
- Toor, I.S.; Rückerl, D.; Mair, I.; Ainsworth, R.; Meloni, M.; Spiroski, A.-M.; Benezech, C.; Felton, J.M.; Thomson, A.; Caporali, A.; et al. Eosinophil Deficiency Promotes Aberrant Repair and Adverse Remodeling Following Acute Myocardial Infarction. JACC Basic. Transl. Sci. 2020, 5, 665–681. [Google Scholar] [CrossRef]
- Davoine, F.; Lacy, P. Eosinophil cytokines, chemokines, and growth factors: Emerging roles in immunity. Front. Immunol. 2014, 5, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puxeddu, I.; Berkman, N.; Nissim Ben Efraim, A.H.; Davies, D.E.; Ribatti, D.; Gleich, G.J.; Levi-Schaffer, F. The role of eosinophil major basic protein in angiogenesis. Allergy 2009, 64, 368–374. [Google Scholar] [CrossRef]
- Jacobsen, E.A.; Taranova, A.G.; Lee, N.A.; Lee, J.J. Eosinophils: Singularly destructive effector cells or purveyors of immunoregulation? J. Allergy Clin. Immunol. 2007, 119, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- McBrien, C.N.; Menzies-Gow, A. The Biology of Eosinophils and Their Role in Asthma. Front. Med. 2017, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra, F.M.; Postma, D.S.; Kauffman, H.F. Mutual activation of pulmonary fibroblasts and eosinophils, and modulation by drugs in relation to asthma. Clin. Exp. Allergy 2001, 31, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Bass, D.A.; Gonwa, T.A.; Szejda, P.; Cousart, M.S.; DeChatelet, L.R.; McCall, C.E. Eosinopenia of acute infection: Production of eosinopenia by chemotactic factors of acute inflammation. J. Clin. Investig. 1980, 65, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Spreng, M. Possible health effects of noise induced cortisol increase. Noise Health 2000, 2, 59–64. [Google Scholar]
- Rao, D.M.; Indrani, G.; RaviKiran, M. Utility of Complete Blood Picture for Predicting In-hospital Mortality in Patients with Acute Decompensated Heart Failure with Dilated Cardiomyopathy. Indian J. Cardiovasc. Dis. Women WINCARS 2017, 2, 44–48. [Google Scholar] [CrossRef]

| All Patients (n = 395) | MACE (+) (n = 176) | MACE (−) (n = 219) | p-Value | |
|---|---|---|---|---|
| Age (years) | 76.5 ± 11.6 | 76.2 ± 10 | 76.8 ± 12.8 | 0.650 |
| Male sex, n (%) | 223 (%56.5) | 82 (%46.5) | 141 (%64.3) | 0.001 |
| BMI (kg/m2) | 25.2 ± 5.4 | 23.4 ± 4.9 | 26.1 ± 5.3 | 0.007 |
| NYHA, n (%) | 0.010 | |||
| Class III | 217 (%54.9) | 62 (%35.2) | 155 (%70.7) | |
| Class IV | 178 (%45.1) | 114 (%64.7) | 64 (%29.2) | |
| AF, n (%) | 227 (%57.5) | 96 (%54.5) | 131 (%59.8) | 0.292 |
| DM, n (%) | 158 (%40) | 79 (%44.8) | 79 (%36.1) | 0.076 |
| HT, n (%) | 324 (%82) | 134 (%76.1) | 190 (%86.7) | 0.006 |
| CAD, n (%) | 287 (%72.7) | 122 (%69.3) | 165 (%75.3) | 0.182 |
| LoS (days) | 7 (4–11) | 11(6–19) | 5 (3–8) | <0.001 |
| Ejection fraction (%) | 31.8 ± 8.5 | 31.1 ± 7.9 | 32.4 ± 8.8 | 0.124 |
| sPAP (mmHg) | 48.6 ± 11.0 | 49.4 ± 10.9 | 48.0 ± 11.1 | 0.365 |
| Leukocytes (109/L) | 9.3 ± 3.4 | 9.4 ± 3.1 | 9.2 ± 3.7 | 0.654 |
| Neutrophil (109/L) | 7.4 ± 3.3 | 7.7 ± 3.0 | 7.1 ± 3.5 | 0.050 |
| Lymphocyte (109/L) | 1.3 ± 0.8 | 1.1 ± 0.6 | 1.4 ± 0.9 | <0.001 |
| Monocyte (109/L) | 0.56 (0.25–0.70) | 0.77 (0.36–1.72) | 0.59 (0.23–0.80) | 0.078 |
| Eosinophil (109/L) | 0.08 ± 0.02 | 0.06 ± 0.01 | 0.10 ± 0.04 | 0.002 |
| Basophil (109/L) | 0.03 ± 0.03 | 0.03 ± 0.02 | 0.04 ± 0.03 | <0.001 |
| Hemoglobin (g/dL) | 12.00 ± 2.01 | 11.76 ± 2.04 | 12.19 ± 1.98 | 0.039 |
| Hematocrit (%) | 37.3 ± 5.9 | 36.8 ± 6.1 | 37.6 ± 5.7 | 0.190 |
| MCV (fL) | 88.1 ± 7.5 | 86.5 ± 7.7 | 89.4 ± 7.1 | <0.001 |
| Platelets (109/L) | 235.9 ± 88.6 | 243.0 ± 98.8 | 230.2 ± 81.2 | 0.158 |
| MPV (fL) | 9.9 ± 1.2 | 9.8 ± 1.2 | 9.9 ± 1.3 | 0.195 |
| Glucose (mg/dL) | 160.4 ± 41.0 | 159.3 ± 48.3 | 161.4 ± 44.9 | 0.800 |
| BUN (mg/dL) | 39.5 ± 12.1 | 45.0 ± 14.2 | 35.1 ± 11.2 | <0.001 |
| ALT (u/L) | 21 (12–37) | 51 (24–101) | 69 (29–119) | 0.165 |
| AST (u/L) | 25 (21–51) | 68 (56–126) | 59 (47–118) | 0.366 |
| Potassium (mmol/L) | 5.0 ± 1.5 | 5.3 ± 1.7 | 4.7 ± 0.6 | 0.196 |
| Sodium (mmol/L) | 137.5 ± 5.6 | 136.5 ± 6.5 | 138.2 ± 4.5 | 0.001 |
| CRP (mg/L) | 12(4.2–25. 8) | 18 (6–39.2) | 26 (12.9–51.7) | 0.016 |
| Albumin (g/dL) | 3.8 ± 0.5 | 3.7 ± 0.5 | 3.8 ± 0.5 | 0.004 |
| Creatinine (mg/dL) | 1.5 ± 0.6 | 1.6 ± 0.7 | 1.4 ± 0.5 | 0.002 |
| NT-proBNP (×103), (pg/mL) | 5.7 (2.9–13.1) | 9.8 (4.4–14.3) | 4.6 (1.9–12.7) | 0.011 |
| Mortality, n (%) | 96 (%24.3) | - | - | - |
| Re-hospitalization, n (%) | 114 (%28.9) | - | - | - |
| MACE, n (%) | 176 (%44.6) | - | - | - |
| NER | 984.1 ± 270.1 | 1527.4 ± 275.7 | 547.5 ± 108.6 | <0.001 |
| LER | 1147.0 ± 335.4 | 1755.9 ± 444.0 | 657.6 ± 124.4 | <0.001 |
| EMR | 0.19 ± 0.05 | 0.17 ± 0.04 | 0.20 ± 0.05 | 0.001 |
| ELR | 0.07 ± 0.02 | 0.06 ± 0.02 | 0.08 ± 0.01 | 0.009 |
| AUC | 95%CI | p-Value | Sensitivity | Specificity | Cut-Off | |
|---|---|---|---|---|---|---|
| NER | 0.699 | 0.641–0.758 | <0.001 | 69% | 64% | 262.00 |
| LER | 0.693 | 0.634–0.753 | <0.001 | 74% | 60% | 264.25 |
| Eosinophil count | 0.693 | 0.632–0.754 | <0.001 | 65% | 66% | 0.025 |
| EMR | 0.663 | 0.602–0.775 | <0.001 | 66% | 64% | 0.064 |
| ELR | 0.660 | 0.597–0.723 | <0.001 | 84% | 45% | 0.057 |
| Neutrophil | 0.637 | 0.574–0.700 | <0.001 | 57% | 69% | 7.785 |
| Lymphocyte | 0.606 | 0.535–0.677 | 0.002 | 45% | 79% | 0.84 |
| Monocyte | 0.538 | 0.472–0.604 | 0.264 | 58% | 53% | 0.555 |
| AUC | 95%CI | p-Value | Sensitivity | Specificity | Cut-Off | |
|---|---|---|---|---|---|---|
| NER | 0.626 | 0.571–0.681 | <0.001 | 76% | 53% | 102.46 |
| LER | 0.616 | 0.560–0.671 | <0.001 | 77% | 51% | 137.02 |
| Eosinophil count | 0.615 | 0.559–0.670 | <0.001 | 67% | 55% | 0.045 |
| EMR | 0.586 | 0.529–0.643 | 0.003 | 70% | 49% | 0.134 |
| ELR | 0.572 | 0.515–0.628 | 0.015 | 72% | 46% | 0.057 |
| Neutrophil | 0.590 | 0.535–0.646 | 0.002 | 81% | 40% | 5.26 |
| Lymphocyte | 0.601 | 0.575–0.687 | <0.001 | 42% | 82% | 0.89 |
| Monocyte | 0.566 | 0.508–0.623 | 0.025 | 35% | 77% | 0.405 |
| Mortality | MACE | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| HR (%95 CI) | p-Value | HR (%95 CI) | p-Value | HR (%95 CI) | p-Value | HR (%95 CI) | p-Value | |
| NER | 3.455 (2.233–5.344) | <0.001 | 3.509 (1.956–6.296) | <0.001 * | 2.657 (1.873–3.770) | <0.001 | 2.740 (1.797–4.177) | <0.001 ** |
| LER | 3.593 (2.277–5.671) | <0.001 | 3.587 (1.969–6.535) | <0.001 * | 2.633 (1.844–3.758) | <0.001 | 2.705 (1.752–4.176) | <0.001 ** |
| EMR | 3.034 (1.984–4.641) | <0.001 | 2.846 (1.641–4.936) | <0.001 * | 1.916 (1.386–2.649) | <0.001 | 1.654 (1.123–2.436) | 0.011 ** |
| ELR | 3.652 (2.104–6.337) | <0.001 | 4.302 (2.064–8.968) | <0.001 * | 1.871 (1.345–2.603) | <0.001 | 2.112 (1.424–3.134) | <0.001 ** |
| Eosinophil | 3.197 (2.097–4.873) | <0.001 | 3.379 (1.916–5.959) | <0.001 * | 2.091 (1.525–2.869) | <0.001 | 1.833 (1.276–2.635) | 0.001 ** |
| Neutrophil | 1.099 (1.043–1.159) | <0.001 | 1.077 (1.001–1.158) | 0.046 * | 1.054 (1.012–1.099) | 0.012 | 1.053 (0.998–1.112) | 0.061 |
| Lymphocyte | 0.671 (0.471–0.957) | 0.028 | 0.726 (0.454–1.161) | 0.181 | 0.600 (0.454–0.794) | <0.001 | 0.822 (0.598–1.129) | 0.226 |
| Monocyte | 0.687 (0.371–1.271) | 0.232 | 1.047 (0.942–1.164) | 0.391 | ||||
| Creatinine | 1.566 (1.162–2.109) | 0.003 | 0.763 (0.494–1.177) | 0.221 | 1.483 (1.176–1.870) | 0.001 | 0.982 (0.719–1.342) | 0.909 |
| Albumin | 0.313 (0.193–0.508) | <0.001 | 0.572 (0.331–0.986) | 0.044 | 0.561 (0.401–0.786) | 0.001 | 0.757 (0.521–1.102) | 0.146 |
| CRP | 1.001 (0.994–1.007) | 0.797 | 0.995 (0.989–1.001) | 0.082 | 0.989 (0.980–1.008) | 0.116 | ||
| Sodium | 0.919 (0.891–0.948) | <0.001 | 0.923 (0.884–0.964) | <0.001 | 0.940 (0.915–0.966) | <0.001 | 0.932 (0.897–0.969) | <0.001 |
| Hemoglobin | 0.860 (0.771–0.960) | 0.007 | 0.896 (0.775–1.036) | 0.139 | 0.909 (0.839–0.985) | 0.020 | 0.946 (0.852–1.050) | 0.298 |
| Hypertension | 0.462 (0.299–0.714) | 0.001 | 0.382 (0.218–0.670) | 0.001 | 0.584 (0.413–0.826) | 0.002 | 0.630 (0.403–1.005) | 0.063 |
| EF | 0.993 (0.970–1.017) | 0.571 | 0.988 (0.971–1.006) | 0.194 | ||||
| Age | 1.001 (0.984–1.018) | 0.904 | 0.998 (0.985–1.010) | 0.785 | ||||
| Hospitalization Period | 1.026 (0.994–1.059) | 0.116 | 1.087 (1.066–1.109) | <0.001 | 1.097 (1.072–1.122) | <0.001 | ||
| NYHA class IV | 1.201 (1.009–2.208) | 0.002 | 1.044 (1.002–1.206) | 0.011 | 1.331 (1.018–2.266) | 0.001 | 1.124 (1.082–1.466) | 0.001 |
| NT-pro-BNP | 1.568 (1.124–2.804) | 0.004 | 1.502 (1.126–2.101) | 0.007 | 1.688 (1.104–2.866) | 0.002 | 1.682 (1.106–2.312) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vural, A.; Aydın, E. The Predictive Value of Eosinophil Indices for Major Cardiovascular Events in Patients with Acute Decompensated HFrEF. Medicina 2022, 58, 1455. https://doi.org/10.3390/medicina58101455
Vural A, Aydın E. The Predictive Value of Eosinophil Indices for Major Cardiovascular Events in Patients with Acute Decompensated HFrEF. Medicina. 2022; 58(10):1455. https://doi.org/10.3390/medicina58101455
Chicago/Turabian StyleVural, Aslı, and Ertan Aydın. 2022. "The Predictive Value of Eosinophil Indices for Major Cardiovascular Events in Patients with Acute Decompensated HFrEF" Medicina 58, no. 10: 1455. https://doi.org/10.3390/medicina58101455





