High-Grade Endometrioid Stromal Sarcoma of the Ovary: Malignant Transformation of Ovarian Mature Cystic Teratoma
Abstract
1. Introduction
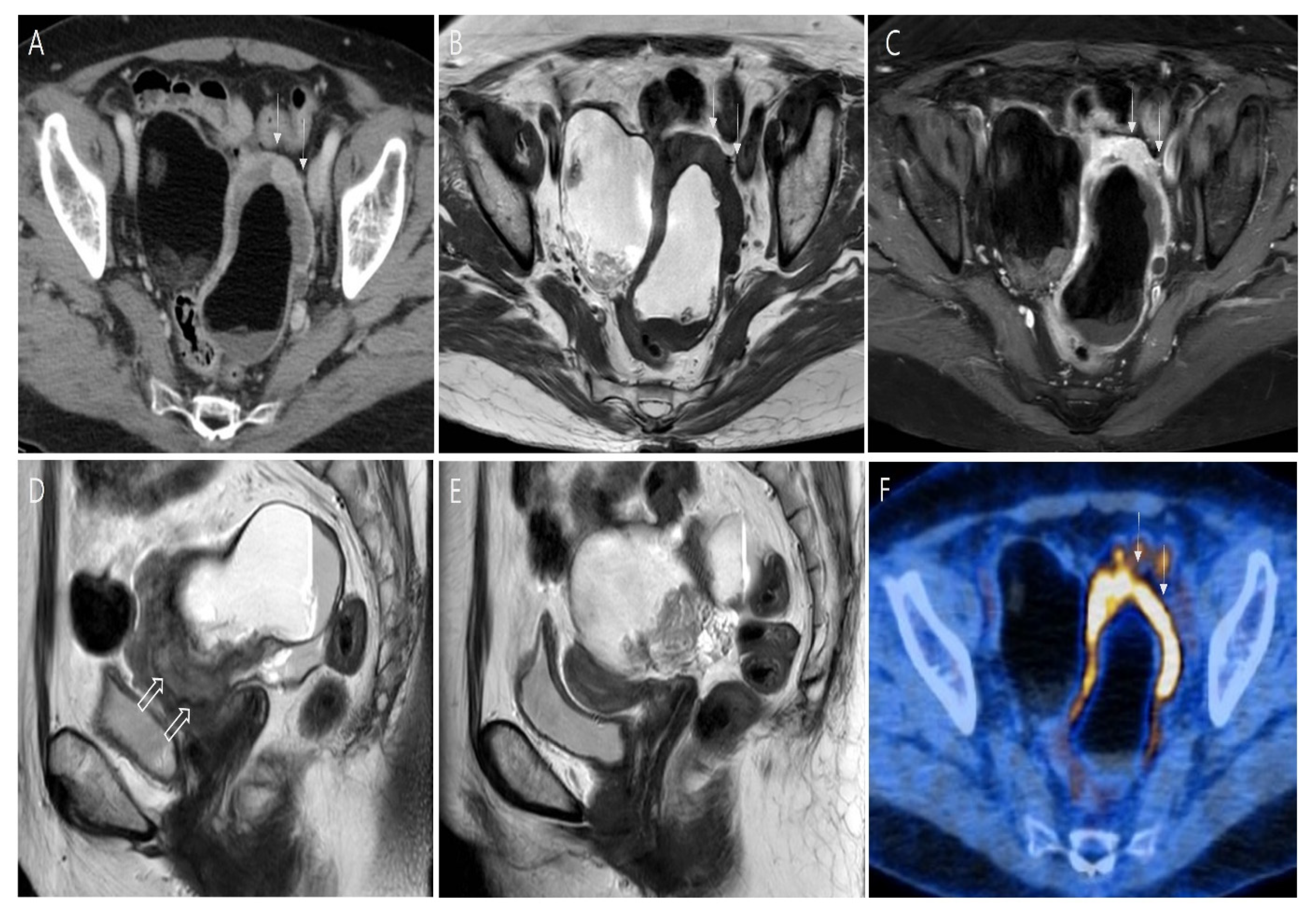
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Classification of Tumours Editorial Boarded. World Health Organization classification of tumours. In Female Genital Tumours, 5th ed.; IARC Press: Lyon, France, 2020; Volume 4, pp. 119–139. [Google Scholar]
- Gadducci, A.; Guerrieri, M.E.; Cosio, S. Squamous cell carcinoma arising from mature cystic teratoma of the ovary: A challenging question for gynecologic oncologists. Crit. Rev. Oncol. Hematol. 2019, 133, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, M.; Shan, Y.; Wu, H.W.; Liu, X.D.; Yin, J.; Gu, Y.; Wang, W.; Wang, Y.X.; Chen, J.Y.; et al. 30-Year Experience With 22 Cases of Malignant Transformation Arising From Ovarian Mature Cystic Teratoma: A Rare Disease. Front. Oncol. 2022, 12, 842703. [Google Scholar] [CrossRef] [PubMed]
- Mahe, E.; Sur, M. Squamous lesions of the ovary. Arch. Pathol. Lab. Med. 2011, 135, 1611–1614. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Q.; Zhang, S.; Dong, R.; Sun, C.; Qiu, C.; Zhang, Z.; Yang, X.; Kong, B. Squamous cell carcinoma transformation in mature cystic teratoma of the ovary: A systematic review. BMC Cancer 2019, 19, 217. [Google Scholar] [CrossRef] [PubMed]
- McCluggage, W.G.; Singh, N.; Gilks, C.B. Key changes to the World Health Organization (WHO) classification of female genital tumours introduced in the 5th edition. Histopathology 2022, 80, 762–778. [Google Scholar] [CrossRef] [PubMed]
- Atwi, D.; Kamal, M.; Quinton, M.; Hassell, L.A. Malignant transformation of mature cystic teratomaof the ovary. J. Obstet. Gynaecol. Res. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Chiang, S.; Ali, R.; Melnyx, N.; McAlpine, J.N.; Huntsman, D.G.; Gilks, C.B.; Lee, C.; Oliva, E. Frequency of known gene rearrangements in endometrial stromal tumors. Am. J. Surg. Pathol. 2011, 35, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Lee, D.; Lee, J.; Han, M.H.; Hong, D.G.; Lee, H.J. Primary ovarian high-grade endometrial stromal sarcoma: A case report. J. Med. Case Rep. 2021, 15, 387. [Google Scholar] [CrossRef] [PubMed]
- Nccn.org [Homepage on the Internet]. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Uterine Cancer. Available online: https://www.nccn.org (accessed on 19 October 2022).
- Dos Santos, L.; Mok, E.; Lasonos, A.; Park, K.; Soslow, R.A.; Aghajanian, C.; Alektiar, K.; Barakat, R.R.; Abu-Rustum, N.R. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: A case series and review of the literature. Gynecol. Oncol. 2007, 105, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Hackethal, A.; Brueggmann, D.; Bohlmann, M.K.; Franke, F.E.; Tinneberg, H.R.; Munstedt, K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: Systematic review and analysis of published data. Lancet Oncol. 2008, 9, 1173–1180. [Google Scholar] [CrossRef]
- Rim, S.Y.; Kim, S.M.; Choi, H.S. Malignant transformation of ovarian mature cystic teratoma. Int. J. Gynecol. Cancer 2006, 16, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Srisajjakul, S.; Prapaisilp, P.; Bangchokdee, S. Imaging features of unusual lesions and complications associated with ovarian mature cystic teratoma. Clin. Imaging 2019, 57, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Buy, J.N.; Ghossain, M.A.; Moss, A.A.; Bazot, M.; Doucet, M.; Hugol, D.; Truc, J.B.; Poitout, P.; Ecoiffier, J. Cystic teratoma of the ovary: CT detection. Radiology 1989, 171, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.; Egger, J.F.; Young, R.H. Primary endometrioid stromal sarcoma of the ovary: A clinicopathologic study of 27 cases with morphologic and behavioral features similar to those of uterine low-grade endometrial stromal sarcoma. Am. J. Surg. Pathol. 2014, 38, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Bacalbasa, N.; Balescu, I.; Dima, S.; Popescu, I. Ovarian sarcoma carries a poorer prognosis than ovarian epithelial cancer throughout all FIGO stages: A single-center case-control matched study. Anticancer Res. 2014, 34, 7303–7308. [Google Scholar] [PubMed]
- Pins, M.R.; Young, R.H.; Daly, W.J.; Scully, R.E. Primary squamous cell carcinoma of the ovary. Report of 37 cases. Am. J. Surg. Pathol. 1996, 20, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, F.; Nawa, A.; Tamakoshi, K.; Ishikawa, H.; Kuzuya, K.; Suganuma, N.; Hattori, S.; Furui, K.; Kawai, M.; Arii, Y. Diagnosis of squamous cell carcinoma arising from mature cystic teratoma of the ovary. Cancer 1998, 82, 2249–2255. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, T.; Liu, X.; Wang, H.; Gu, X.; Chen, D.; Wang, Z.; He, D. Malignant transformation arising from mature ovarian cystic teratoma: A case series. Medicine 2021, 100, e24726. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Miller, D.M.; Ehlen, T.G. Peritoneal implantation of squamous cell carcinoma following rupture of a dermoid cyst during laparoscopic removal. Gynecol. Oncol. 2002, 84, 180–183. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Baek, J.C. High-Grade Endometrioid Stromal Sarcoma of the Ovary: Malignant Transformation of Ovarian Mature Cystic Teratoma. Medicina 2022, 58, 1501. https://doi.org/10.3390/medicina58101501
Kim H, Baek JC. High-Grade Endometrioid Stromal Sarcoma of the Ovary: Malignant Transformation of Ovarian Mature Cystic Teratoma. Medicina. 2022; 58(10):1501. https://doi.org/10.3390/medicina58101501
Chicago/Turabian StyleKim, Hyoeun, and Jong Chul Baek. 2022. "High-Grade Endometrioid Stromal Sarcoma of the Ovary: Malignant Transformation of Ovarian Mature Cystic Teratoma" Medicina 58, no. 10: 1501. https://doi.org/10.3390/medicina58101501
APA StyleKim, H., & Baek, J. C. (2022). High-Grade Endometrioid Stromal Sarcoma of the Ovary: Malignant Transformation of Ovarian Mature Cystic Teratoma. Medicina, 58(10), 1501. https://doi.org/10.3390/medicina58101501





