The Beneficial Effect of Personalized Lifestyle Intervention in Chronic Kidney Disease Follow-Up Project for National Health Insurance Specific Health Checkup: A Five-Year Community-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects and Intervention
2.2. Baseline Measurements
2.3. Statistics
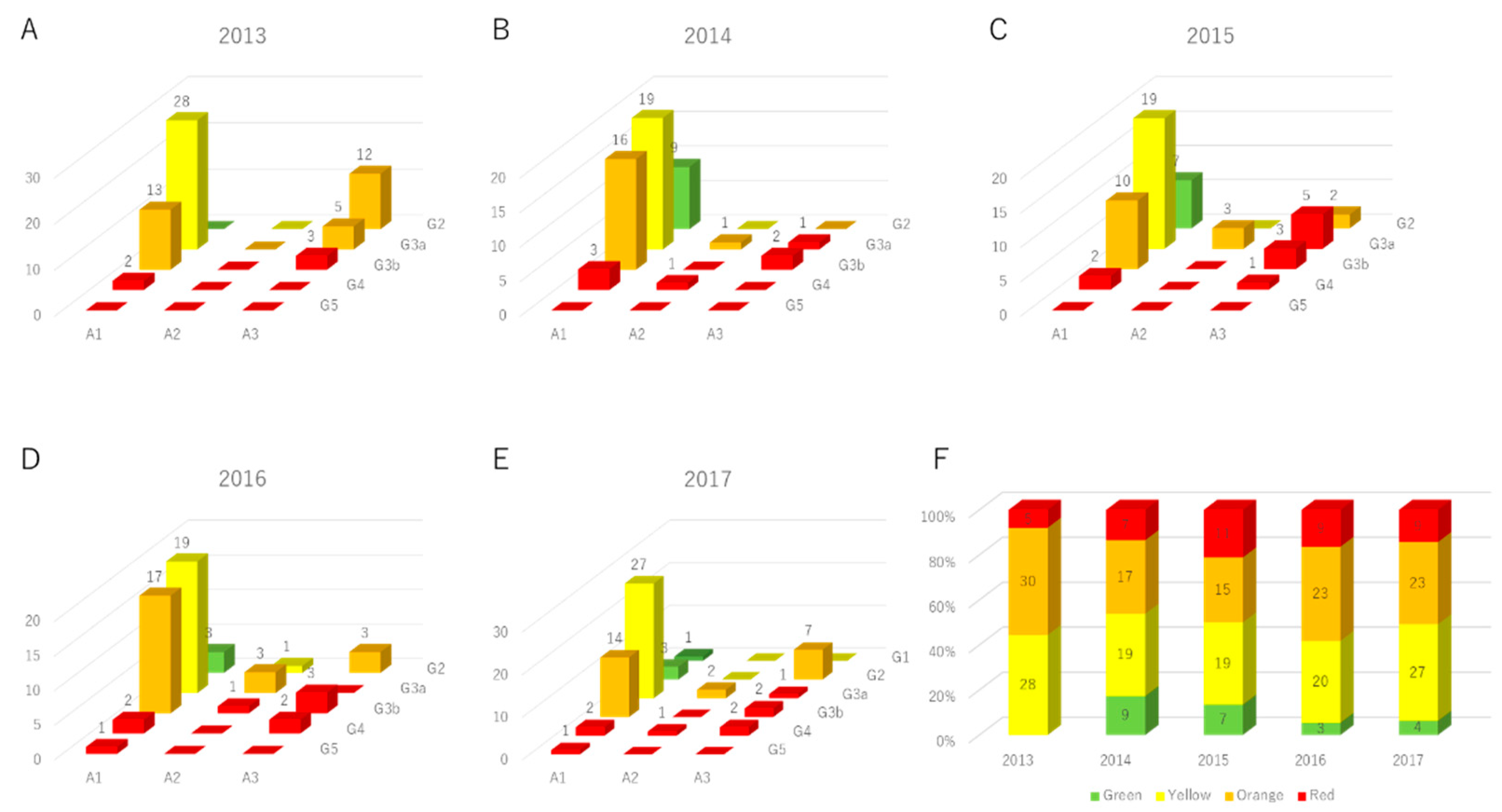
3. Results
3.1. Study Participants
3.2. The Parameter Changes in High-Risk Subjects for Advanced Renal Dysfunction
3.3. The Effect of Medical Institution Visit on Renal Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagata, M.; Ninomiya, T.; Doi, Y.; Yonemoto, K.; Kubo, M.; Hata, J.; Tsuruya, K.; Iida, M.; Kiyohara, Y. Trends in the prevalence of chronic kidney disease and its risk factors in a general Japanese population: The Hisayama Study. Nephrol. Dial. Transpl. 2010, 25, 2557–2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, E.; Horio, M.; Watanabe, T.; Iseki, K.; Yamagata, K.; Hara, S.; Ura, N.; Kiyohara, Y.; Moriyama, T.; Ando, Y.; et al. Prevalence of chronic kidney disease in the Japanese general population. Clin. Exp. Nephrol. 2009, 13, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Van Der Velde, M.; Astor, B.C.; Woodward, M.; Levey, A.S.; De Jong, P.E.; Coresh, J.; Gansevoort, R.T. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 2010, 375, 2073–2081. [Google Scholar] [CrossRef] [Green Version]
- Astor, B.C.; The Chronic Kidney Disease Prognosis Consortium; Matsushita, K.; Gansevoort, R.T.; van der Velde, M.; Woodward, M.; Levey, A.S.; de Jong, P.E.; Coresh, J. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney. Int. 2011, 79, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Hori, K.; Saito, O.; Hashimoto, S.; Matsui, T.; Akter, R.; Takeuchi, K. Projecting population distribution under depopulation conditions in Japan: Scenario analysis for future socio-ecological systems. Sustain. Sci. 2020, 16, 1–17. [Google Scholar]
- Sofue, T.; Kagawa Association of Chronic Kidney Disease Initiatives; Okano, Y.; Matsushita, N.; Moritoki, M.; Nishijima, Y.; Fujioka, H.; Yamasaki, Y.; Yamanaka, M.; Nishiyama, A.; et al. The effects of a participatory structured group educational program on the development of CKD: A population-based study. Clin. Exp. Nephrol. 2019, 23, 1031–1038. [Google Scholar] [CrossRef]
- Byrne, J.; Khunti, K.; Stone, M.; Farooqi, A.; Carr, S. Feasibility of a structured group education session to improve self-management of blood pressure in people with chronic kidney disease: An open randomised pilot trial. BMJ Open 2011, 1, e000381. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.; Khunti, K.; Stone, M.; Farooqi, A.; Carr, S. Educational Interventions in Kidney Disease Care: A Systematic Review of Randomized Trials. Am. J. Kidney Dis. 2008, 51, 933–951. [Google Scholar] [CrossRef]
- Yamagata, K.; Makino, H.; Iseki, K.; Ito, S.; Kimura, K.; Kusano, E.; Shibata, T.; Tomita, K.; Narita, I.; Nishino, T.; et al. Effect of Behavior Modification on Outcome in Early- to Moderate-Stage Chronic Kidney Disease: A Cluster-Randomized Trial. PLoS ONE 2016, 11, e0151422. [Google Scholar] [CrossRef] [Green Version]
- Yamagishi, K.; Iso, H. The criteria for metabolic syndrome and the national health screening and education system in Japan. Epidemiol. Health 2017, 39, e2017003. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare in Japan. The Health Guidance Program. 2007; pp. 1–252. Available online: http://www.mhlw.go.jp/bunya/kenkou/seikatsu/pdf/02 (accessed on 21 April 2007).
- Wachtell, K.; Ibsen, H.; Olsen, M.H.; Borch-Johnsen, K.; Lindholm, L.H.; Mogensen, C.E.; Dahlöf, B.; Devereux, R.B.; Beevers, G.; De Faire, U.; et al. Albuminuria and Cardiovascular Risk in Hypertensive Patients with Left Ventricular Hypertrophy: The LIFE Study. Ann. Intern. Med. 2003, 139, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.J.G. Japan nephrology, s., [Special issue: Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012]. Jpn. J. Nephrol. 2012, 54, 1034–1191. [Google Scholar]
- Nagai, K.; Yamagata, K.; Iseki, K.; Moriyama, T.; Tsuruya, K.; Fujimoto, S.; Narita, I.; Konta, T.; Kondo, M.; Kasahara, M.; et al. Antihypertensive treatment and risk of cardiovascular mortality in patients with chronic kidney disease diagnosed based on the presence of proteinuria and renal function: A large longitudinal study in Japan. PLoS ONE 2019, 14, e0225812. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Kakio, Y.; Uchida, H.A.; Takeuchi, H.; Okuyama, Y.; Umebayashi, R.; Watatani, H.; Maeshima, Y.; Sugiyama, H.; Wada, J. Report of health checkup system for chronic kidney disease in general population in Okayama city: Effect of health guidance intervention on chronic kidney disease outcome. Int. J. Nephrol. Renov. Dis. 2019, 12, 143–152. [Google Scholar] [CrossRef] [Green Version]
- Inaguma, D.; For The Chronic Kidney Disease Japan Cohort Study Group; Imai, E.; Takeuchi, A.; Ohashi, Y.; Watanabe, T.; Nitta, K.; Akizawa, T.; Matsuo, S.; Makino, H.; et al. Risk factors for CKD progression in Japanese patients: Findings from the Chronic Kidney Disease Japan Cohort (CKD-JAC) Study. Clin. Exp. Nephrol. 2016, 21, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Takei, T.; Shirota, S.; Tsukada, M.; Sugiura, H.; Itabashi, M.; Ogawa, T.; Uchida, K.; Tsuchiya, K.; Nitta, K. Risk Factors for Progression in Patients with Early-stage Chronic Kidney Disease in the Japanese Population. Intern. Med. 2008, 47, 1859–1864. [Google Scholar] [CrossRef] [Green Version]
- McMahon, E.J.; Bauer, J.D.; Hawley, C.M.; Isbel, N.M.; Stowasser, M.; Johnson, D.W.; Campbell, K.L. A Randomized Trial of Dietary Sodium Restriction in CKD. J. Am. Soc. Nephrol. 2013, 24, 2096–2103. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, T.; Liu, Y.; Crews, D.C. Dietary Patterns and CKD Progression. Blood Purif. 2016, 41, 117–122. [Google Scholar] [CrossRef]
- Delgado, C.; Baweja, M.; Crews, D.C.; Eneanya, N.D.; Gadegbeku, C.A.; Inker, L.A.; Mendu, M.L.; Miller, W.G.; Moxey-Mims, M.M.; Roberts, G.V.; et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am. J. Kidney Dis. 2022, 79, 268–288.e1. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Eneanya, N.D.; Coresh, J.; Tighiouart, H.; Wang, D.; Sang, Y.; Crews, D.C.; Doria, A.; Estrella, M.M.; Froissart, M.; et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. N. Engl. J. Med. 2021, 385, 1737–1749. [Google Scholar] [CrossRef] [PubMed]




| Gender | Male 1102/Female 1274 (Ratio: 0.46) |
|---|---|
| Age, (years) | 65.0 ± 7.2 |
| 40–49 years old, n (%) | 117 (4.9%) |
| 50–59 years old, n (%) | 244 (10.3 %) |
| 60–69 years old, n (%) | 1255 (52.8 %) |
| 70–74 years old, n (%) | 760 (32.0 %) |
| Urine protein | |
| No data, n (%) | 1 |
| −, n (%) | 2249 (94.7 %) |
| ±, n (%) | 74 (3.1 %) |
| +, n (%) | 34 (1.4 %) |
| 2+, n (%) | 15 (0.6 %) |
| 3+, n (%) | 3 (0.1 %) |
| Renal function | |
| Creatinine, (mg/dL) | 0.74 ± 0.29 |
| eGFR, (mL/min/1.73 m2) | 75.2 ± 15.4 |
| Variable | 2013 (n = 63) | 2014 (n = 54) | 2015 (n = 52) | 2016 (n = 55) | 2017 (n = 63) | p Value |
|---|---|---|---|---|---|---|
| Age, (years) | 67.2 ± 5.0 | |||||
| Gender (male), n (%) | 36 (56.3 %) | |||||
| Systolic blood pressure | 133 ± 16 | 131 ± 16 | 130 ± 16 | 131 ± 19 | 135 ± 19 | 0.546 |
| Diastolic blood pressure | 77 ± 11 | 76 ± 10 | 74 ± 11 | 77 ± 12 | 77 ± 11 | 0.657 |
| Body mass index, (kg/m²) | 23.8 ± 3.4 | 23.3 ± 3.0 | 23.6 ± 3.4 | 23.3 ± 3.5 | 23.6 ± 3.5 | 0.207 |
| Uric acid, (mg/dL) | 6.3 ± 1.6 | 6.3 ± 1.7 | 6.0 ± 1.4 | 5.9 ± 1.3 | 6.0 ± 1.3 | 0.084 |
| Triglyceride, (mg/dL) | 115 ± 59 | 113 ± 47 | 115 ± 53 | 113 ± 51 | 127 ± 82 | 0.171 |
| HDL cholesterol, (mg/dL) | 57 ± 15 | 61 ± 15 | 59 ± 14 | 58 ± 15 | 54 ± 15 | 0.033 * |
| LDL cholesterol, (mg/dL) | 121 ± 29 | 122 ± 27 | 123 ± 26 | 117 ± 26 | 114 ± 30 | 0.059 |
| AST, (IU/L) | 23 ± 9 | 23 ± 6 | 23 ± 7 | 24 ± 9 | 24 ± 12 | 0.355 |
| ALT, (IU/L) | 18 ± 11 | 18 ± 7 | 18 ± 9 | 18 ± 10 | 18 ± 10 | 0.902 |
| γ-GTP, (IU/L) | 28 ± 20 | 29 ± 21 | 29 ± 23 | 28 ± 18 | 32 ± 32 | 0.169 |
| HbA1c, (%) | 5.8 ± 0.6 | 5.8 ± 0.5 | 5.8 ± 0.5 | 5.8 ± 0.6 | 5.8 ± 0.7 | 0.457 |
| Creatinine, (mg/dL) | 1.08 ± 0.32 | 1.14 ± 0.40 | 1.10 ± 0.37 | 1.19 ± 0.51 | 1.16 ± 0.56 | 0.047 * |
| eGFR, (mL/min/1.73 m²) | 50.7 ± 13.6 | 48.2 ± 13.6 | 49.6 ± 12.7 | 46.7 ± 13.7 | 49.1 ± 15.7 | 0.068 |
| Urine protein | ||||||
| −, n (%) | 43 (68.3 %) | 48 (88.9 %) | 38 (73.1 %) | 42 (76.4 %) | 51 (81.0 %) | 0.034 * |
| ±, n (%) | 0 (0 %) | 2 (3.7 %) | 4 (7.7 %) | 5 (9.1 %) | 3 (4.8 %) | |
| +, n (%) | 16 (25.4 %) | 3 (5.6 %) | 7 (13.5 %) | 5 (9.1 %) | 3 (4.8 %) | |
| 2+, n (%) | 3 (4.8 %) | 0 (0 %) | 2 (3.8 %) | 2 (3.6 %) | 5 (7.9 %) | |
| 3+, n (%) | 1 (1.6 %) | 1 (1.9 %) | 1 (1.9 %) | 1 (1.8 %) | 1 (1.6 %) |
| Variables | B | SE | β | t Value | p Value |
|---|---|---|---|---|---|
| Age (10 years) | 4.753 | 1.574 | 0.341 | 3.019 | 0.004 ** |
| Male (1/0) | −1.229 | 1.793 | −0.087 | −0.685 | 0.496 |
| Current Smoker (1/0) | 5.878 | 2.645 | 0.295 | 2.222 | 0.031 * |
| Drinking Habits (1/0) | 3.522 | 1.937 | 0.210 | 1.818 | 0.075 |
| Medical Institution Visit (1/0) | 0.953 | 1.625 | 0.066 | 0.586 | 0.560 |
| History of Heart Disease (1/0) | 0.850 | 3.155 | 0.030 | 0.270 | 0.789 |
| History of Stroke (1/0) | 1.018 | 2.516 | 0.043 | 0.405 | 0.687 |
| Hypertension (1/0) | −1.893 | 1.561 | −0.134 | −1.213 | 0.231 |
| Dyslipidemia (1/0) | −0.355 | 1.535 | −0.025 | −0.231 | 0.818 |
| Diabetes (1/0) | −2.754 | 3.009 | −0.116 | −0.915 | 0.364 |
| Hyperuricemia (1/0) | −0.511 | 1.792 | −0.035 | −0.285 | 0.777 |
| Baseline eGFR (/10 mL/min/1.73 m²) | −1.979 | 0.723 | −0.383 | −2.736 | 0.009 ** |
| Urine protein (−: 0, ±: 1, 1+: 2, 2+: 3, 3+: 4) | 4.503 | 0.987 | 0.705 | 4.560 | <0.001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeuchi, H.; Uchida, H.A.; Katayama, K.; Matsuoka-Uchiyama, N.; Okamoto, S.; Onishi, Y.; Okuyama, Y.; Umebayashi, R.; Miyaji, K.; Kai, A.; et al. The Beneficial Effect of Personalized Lifestyle Intervention in Chronic Kidney Disease Follow-Up Project for National Health Insurance Specific Health Checkup: A Five-Year Community-Based Cohort Study. Medicina 2022, 58, 1529. https://doi.org/10.3390/medicina58111529
Takeuchi H, Uchida HA, Katayama K, Matsuoka-Uchiyama N, Okamoto S, Onishi Y, Okuyama Y, Umebayashi R, Miyaji K, Kai A, et al. The Beneficial Effect of Personalized Lifestyle Intervention in Chronic Kidney Disease Follow-Up Project for National Health Insurance Specific Health Checkup: A Five-Year Community-Based Cohort Study. Medicina. 2022; 58(11):1529. https://doi.org/10.3390/medicina58111529
Chicago/Turabian StyleTakeuchi, Hidemi, Haruhito A. Uchida, Katsuyoshi Katayama, Natsumi Matsuoka-Uchiyama, Shugo Okamoto, Yasuhiro Onishi, Yuka Okuyama, Ryoko Umebayashi, Kodai Miyaji, Akiko Kai, and et al. 2022. "The Beneficial Effect of Personalized Lifestyle Intervention in Chronic Kidney Disease Follow-Up Project for National Health Insurance Specific Health Checkup: A Five-Year Community-Based Cohort Study" Medicina 58, no. 11: 1529. https://doi.org/10.3390/medicina58111529





