Fecal Microbiota Transplantation in NAFLD Treatment
Abstract
1. Introduction
2. Materials and Methods
3. Results
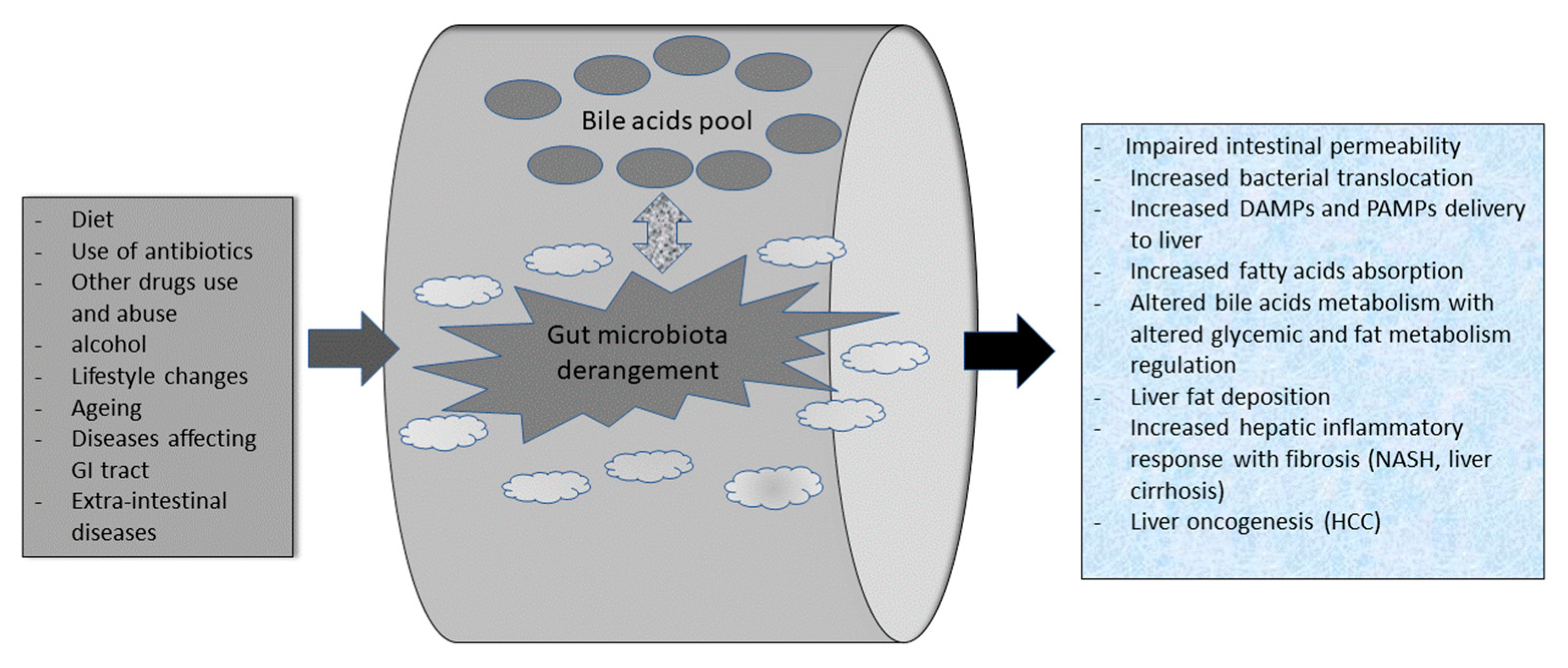
3.1. Gut Microbiota Composition in Health and Its “dysbiosis” in NAFLD Patients
3.2. Bile Acids (BA), Gut Microbiota, and NAFLD
3.3. From Probiotics Use to Fecal Microbiota Transplantation (FMT) in NALFD Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Hofman, D.; Kudla, U.; Miqdady, M.; Nguyen, T.V.H.; Morán-Ramos, S.; Vandenplas, Y. Faecal Microbiota in Infants and Young Children with Functional Gastrointestinal Disorders: A Systematic Review. Nutrients 2022, 14, 974. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, K.N.; Matukane, S.R.; Hamman, B.L.; Whitelaw, A.C.; Newton-Foot, M. Effect of antibiotics on the human microbiome: A systematic review. Int. J. Antimicrob. Agents 2021, 59, 106502. [Google Scholar] [CrossRef]
- Thrastardottir, T.O.; Copeland, V.J.; Constantinou, C. The Association Between the Gut Microbiome, Nutritional Habits, Antibiotics, and Gastric Cancer: A Scoping Review. Curr. Nutr. Rep. 2022, 11, 19–38. [Google Scholar] [CrossRef]
- Amedei, A.; Boem, F. I’ve Gut A Feeling: Microbiota Impacting the Conceptual and Experimental Perspectives of Personalized Medicine. Int. J. Mol. Sci. 2018, 19, 3756. [Google Scholar] [CrossRef] [PubMed]
- Yaqub, S.; Ananias, P.; Shah, A.; Luenam, K.; Jose, A.M.; Melo, J.P.; Turkistani, A.; Mohammed, L. Decoding the Pathophysiology of Non-alcoholic Fatty Liver Disease Progressing to Non-alcoholic Steatohepatitis: A Systematic Review. Cureus 2021, 13, e18201. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Mascaró, C.M.; Bouzas, C.; A Tur, J. Association between Non-Alcoholic Fatty Liver Disease and Mediterranean Lifestyle: A Systematic Review. Nutrients 2021, 14, 49. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 21, S1542–S3565. [Google Scholar] [CrossRef]
- Bifari, F.; Manfrini, R.; Cas, M.D.; Berra, C.; Siano, M.; Zuin, M.; Paroni, R.; Folli, F. Multiple target tissue effects of GLP-1 analogues on non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Pharmacol. Res. 2018, 137, 219–229. [Google Scholar] [CrossRef]
- Majzoub, A.M.; Nayfeh, T.; Barnard, A.; Munaganuru, N.; Dave, S.; Singh, S.; Murad, M.H.; Loomba, R. Systematic review with network meta-analysis: Comparative efficacy of pharmacologic therapies for fibrosis improvement and resolution of NASH. Aliment. Pharmacol. Ther. 2021, 54, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Manara, M.; Colombo, S.; De Santi, G.; Ghidini, M.; Mariani, M.; Iaculli, A.; Rausa, E.; Rampulla, V.; Arru, M.; et al. Hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis: HCC and Steatosis or Steatohepatitis. Neoplasia 2022, 30, 100809. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ye, J.; Shao, C.; Zhong, B. Compositional alterations of gut microbiota in nonalcoholic fatty liver disease patients: A systematic review and Meta-analysis. Lipids Health Dis. 2021, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- De Munck, T.J.I.; Xu, P.; Verwijs, H.J.A.; Masclee, A.A.M.; Jonkers, D.; Verbeek, J.; Koek, G.H. Intestinal permeability in human nonalcoholic fatty liver disease: A systematic review and meta-analysis. Liver Int. 2020, 40, 2906–2916. [Google Scholar] [CrossRef]
- Stofan, M.; Guo, G.L. Bile Acids and FXR: Novel Targets for Liver Diseases. Front. Med. 2020, 7, 544. [Google Scholar] [CrossRef]
- Mori, H.; Baroni, G.S.; Marzioni, M.; Di Nicola, F.; Santori, P.; Maroni, L.; Abenavoli, L.; Scarpellini, E. Farnesoid X Receptor, Bile Acid Metabolism, and Gut Microbiota. Metabolites 2022, 12, 647. [Google Scholar] [CrossRef]
- Sabirin, F.; Lim, S.M.; Neoh, C.F.; Ramasamy, K. Hepatoprotection of Probiotics Against Non-Alcoholic Fatty Liver Disease in vivo: A Systematic Review. Front. Nutr. 2022, 9, 844374. [Google Scholar] [CrossRef]
- Rakotonirina, A.; Galperine, T.; Allémann, E. Fecal microbiota transplantation: A review on current formulations in Clostridioides difficile infection and future outlooks. Expert Opin. Biol. Ther. 2022, 22, 929–944. [Google Scholar] [CrossRef]
- Suk, K.T.; Koh, H. New perspective on fecal microbiota transplantation in liver diseases. J. Gastroenterol. Hepatol. 2021, 37, 24–33. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Scarpellini, E.; Fagoonee, S.; Rinninella, E.; Rasetti, C.; Aquila, I.; LaRussa, T.; Ricci, P.; Luzza, F.; Abenavoli, L. Gut Microbiota and Liver Interaction through Immune System Cross-Talk: A Comprehensive Review at the Time of the SARS-CoV-2 Pandemic. J. Clin. Med. 2020, 9, 2488. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Taylor, T.; Ohno, H.; Hattori, M. Acetate-producing bifidobacteria protect the host from enteropathogenic infection via carbohydrate transporters. Gut Microbes 2012, 3, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, E.; Basilico, M.; Rinninella, E.; Carbone, F.; Schol, J.; Rasetti, C.; Abenavoli, L.; Santori, P. Probiotics and gut health. Minerva Gastroenterol. 2022, 67, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826–2832. [Google Scholar] [CrossRef] [PubMed]
- Melbye, P.; Olsson, A.; Hansen, T.H.; Søndergaard, H.B.; Oturai, A.B. Short-chain fatty acids and gut microbiota in multiple sclerosis. Acta Neurol. Scand. 2018, 139, 208–219. [Google Scholar] [CrossRef]
- Schaad, U.B.; Mütterlein, R.; Goffin, H.; BV-Child Study Group. Immunostimulation with OM-85 in Children with Recurrent Infections of the Upper Respiratory Tract: A Double-Blind, Placebo-Controlled Multicenter Study. Chest 2002, 122, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Feleszko, W.; Jaworska, J.; Rha, R.-D.; Steinhausen, S.; Avagyan, A.; Jaudszus, A.; Ahrens, B.; Groneberg, D.A.; Wahn, U.; Hamelmann, E. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin. Exp. Allergy 2006, 37, 498–505. [Google Scholar] [CrossRef]
- Jadhav, K.; Cohen, T.S. Can You Trust Your Gut? Implicating a Disrupted Intestinal Microbiome in the Progression of NAFLD/NASH. Front. Endocrinol. 2020, 11, 592157. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, N.; Wang, X.; Chi, Y.; Zhang, Y.; Qiu, X.; Hu, Y.; Li, J.; Liu, Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 08096. [Google Scholar] [CrossRef]
- Del Chierico, F.; Nobili, V.; Vernocchi, P.; Russo, A.; De Stefanis, C.; Gnani, D.; Furlanello, C.; Zandonà, A.; Paci, P.; Capuani, G.; et al. Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Burz, S.; Monnoye, M.; Philippe, C.; Farin, W.; Ratziu, V.; Strozzi, F.; Paillarse, J.-M.; Chêne, L.; Blottière, H.; Gérard, P. Fecal Microbiota Transplant from Human to Mice Gives Insights into the Role of the Gut Microbiota in Non-Alcoholic Fatty Liver Disease (NAFLD). Microorganisms 2021, 9, 199. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Al-Hassi, H.O.; Steed, H.; Phipps, O.; Brookes, M.J. Bile Acids and the Microbiome: Making Sense of This Dynamic Relationship in Their Role and Management in Crohn’s Disease. Can. J. Gastroenterol. Hepatol. 2022, 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cowen, A.E.; Κοrman, M.G.; Hofmann, A.F.; Cass, O.W.; Coffin, S.B. Metabolism of Lithocholate in Healthy Man. II. Entero-hepatic circulation. Gastroenterology 1975, 69, 67–76. [Google Scholar] [CrossRef]
- Urdaneta, V.; Casadesús, J. Interactions between Bacteria and Bile Salts in the Gastrointestinal and Hepatobiliary Tracts. Front. Med. 2017, 4, 163. [Google Scholar] [CrossRef]
- Kumar, R.S.; Brannigan, J.A.; Prabhune, A.; Pundle, A.V.; Dodson, G.G.; Dodson, E.J.; Suresh, C.G. Structural and Functional Analysis of a Conjugated Bile Salt Hydrolase from Bifidobacterium longum Reveals an Evolutionary Relationship with Penicillin V Acylase. J. Biol. Chem. 2006, 281, 32516–32525. [Google Scholar] [CrossRef]
- Gopal-Srivastava, R.; Hylemon, P.B. Purification and characterization of bile salt hydrolase from Clostridium perfringens. J. Lipid Res. 1988, 29, 1079–1085. [Google Scholar] [CrossRef]
- Chand, D.; Panigrahi, P.; Varshney, N.; Ramasamy, S.; Suresh, C. Structure and function of a highly active Bile Salt Hydrolase (BSH) from Enterococcus faecalis and post-translational processing of BSH enzymes. Biochim. et Biophys. Acta (BBA)-Proteins Proteom. 2018, 1866, 507–518. [Google Scholar] [CrossRef]
- Stellwag, E.; Hylemon, P. Purification and characterization of bile salt hydrolase from Bacteroides fragilis subsp. fragilis. Biochim. et Biophys. Acta (BBA)-Enzym. 1976, 452, 165–176. [Google Scholar] [CrossRef]
- Begley, M.; Gahan, C.G.; Hill, C. The interaction between bacteria and bile. FEMS Microbiol. Rev. 2005, 29, 625–651. [Google Scholar] [CrossRef]
- Dussurget, O.; Cabanes, D.; Dehoux, P.; Lecuit, M.; Buchrieser, C.; Glaser, P.; Cossart, P. The European Listeria Genome Consortium Listeria monocytogenes bile salt hydrolase is a PrfA-regulated virulence factor involved in the intestinal and hepatic phases of listeriosis. Mol. Microbiol. 2002, 45, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Park, D.; Chun, B.; Hahn, Y.; Jeon, C. Diet-Related Alterations of Gut Bile Salt Hydrolases Determined Using a Metagenomic Analysis of the Human Microbiome. Int. J. Mol. Sci. 2021, 22, 3652. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Van Hoorde, L.; Woestyne, M.V.; Christiaens, H.; Verstraete, W. Significance of bile salt hydrolytic activities of lactobacilli. J. Appl. Bacteriol. 1995, 79, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, A.; Fiorotto, R.; Strazzabosco, M. Bile acids and their receptors: Modulators and therapeutic targets in liver inflammation. Semin. Immunopathol. 2022, 44, 547–564. [Google Scholar] [CrossRef]
- Parséus, A.; Sommer, N.; Sommer, F.; Caesar, R.; Molinaro, A.; Ståhlman, M.; Greiner, T.U.; Perkins, R.; Bäckhed, F. Microbiota-induced obesity requires farnesoid X receptor. Gut 2017, 66, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Pineda Torra, I.; Claudel, T.; Duval, C.; Kosykh, V.; Fruchart, J.-C.; Staels, B. Bile Acids Induce the Expression of the Human Peroxisome Proliferator-Activated Receptor Alpha Gene via Activation of the Farnesoid X Receptor. Mol. Endocrinol. 2003, 17, 259–272. [Google Scholar] [CrossRef]
- Penney, N.; Kinross, J.; Newton, R.C.; Purkayastha, S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: A systematic review. Int. J. Obes. 2015, 39, 1565–1574. [Google Scholar] [CrossRef]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- He, S.; Cui, S.; Song, W.; Jiang, Y.; Chen, H.; Liao, D.; Lu, X.; Li, J.; Chen, X.; Peng, L. Interleukin-17 Weakens the NAFLD/NASH Process by Facilitating Intestinal Barrier Restoration Depending on the Gut Microbiota. mBio 2022, 13, e0368821. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Mykhalchyshyn, G.; Kononenko, L.; Boccuto, L.; Kyriienko, D.; Dynnyk, O. A Multi-strain Probiotic Reduces the Fatty Liver Index, Cytokines and Aminotransferase levels in NAFLD Patients: Evidence from a Randomized Clinical Trial. J. Gastrointest. Liver Dis. 2018, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Guasti, L.; D’Angelo, A.; Martinotti, C.; Valentino, M.C.; Di Matteo, S.; Bruno, G.M.; Maresca, A.M.; Gaudio, G.V.; Maffioli, P. Probiotic Therapy With VSL#3® in Patients With NAFLD: A Randomized Clinical Trial. Front Nutr. 2022, 9, 846873. [Google Scholar] [CrossRef] [PubMed]
- Lirussi, F.; Mastropasqua, E.; Orando, S.; Orlando, R. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst. Rev. 2007, 2007, CD005165. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Federico, A.; Tuccillo, C.; Terracciano, F.; D’Auria, M.V.; De Simone, C.; Del Vecchio Blanco, C. Beneficial Effects of a Probiotic VSL#3 on Parameters of Liver Dysfunction in Chronic Liver Diseases. J. Clin. Gastroenterol. 2005, 39, 540–543. [Google Scholar] [CrossRef]
- Vajro, P.; Mandato, C.; Licenziati, M.R.; Franzese, A.; Vitale, D.F.; Lenta, S.; Caropreso, M.; Vallone, G.; Meli, R. Effects of Lactobacillus rhamnosus Strain GG in Pediatric Obesity-related Liver Disease. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; De Luis, D.A.; Izaola, O.; Conde, R.; Gonzalez Sagrado, M.; Primo, D.; De La Fuente, B.; Gonzalez, J. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: A double blind randomized clinical trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 1090–1095. [Google Scholar]
- Alisi, A.; Bedogni, G.; Baviera, G.; Giorgio, V.; Porro, E.; Paris, C.; Giammaria, P.; Reali, L.; Anania, F.; Nobili, V. Randomised clinical trial: The beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment. Pharmacol. Ther. 2014, 39, 1276–1285. [Google Scholar] [CrossRef]
- Famouri, F.; Shariat, Z.; Hashemipour, M.; Keikha, M.; Kelishadi, R. Effects of Probiotics on Nonalcoholic Fatty Liver Disease in Obese Children and Adolescents. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 413–417. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Tse, C.-H.; Lam, T.-T.Y.; Wong, G.L.-H.; Chim, A.M.-L.; Chu, W.C.-W.; Yeung, D.K.-W.; Law, P.T.-W.; Kwan, H.S.; Yu, J.; et al. Molecular Characterization of the Fecal Microbiota in Patients with Nonalcoholic Steatohepatitis–A Longitudinal Study. PLoS ONE 2013, 8, e62885. [Google Scholar] [CrossRef]
- Kobyliak, N.; Abenavoli, L.; Falalyeyeva, T.; Mykhalchyshyn, G.; Boccuto, L.; Kononenko, L.; Kyriienko, D.; Komisarenko, I.; Dynnyk, O. Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: A randomized clinical study. Minerva Med. 2018, 109, 418–428. [Google Scholar] [CrossRef]
- Wang, W.; Shi, L.P.; Shi, L.; Xu, L. [Efficacy of probiotics on the treatment of non-alcoholic fatty liver disease]. Zhonghua Nei Ke Za Zhi 2018, 57, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Manzhalii, E.; Virchenko, O.; Falalyeyeva, T.; Beregova, T.; Stremmel, W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J. Dig. Dis. 2017, 18, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Sepideh, A.; Karim, P.; Hossein, A.; Leila, R.; Hamdollah, M.; Mohammad, E.G.; Mojtaba, S.; Mohammad, S.; Ghader, G.; Moayed, A.S. Effects of Multistrain Probiotic Supplementation on Glycemic and Inflammatory Indices in Patients with Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. J. Am. Coll. Nutr. 2015, 35, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.; Rafraf, M.; Somi, M.; Homayouni-Rad, A.; Asghari-Jafarabadi, M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J. Dairy Sci. 2014, 97, 7386–7393. [Google Scholar] [CrossRef]
- Le Roy, T.; Llopis, M.; Lepage, P.; Bruneau, A.; Rabot, S.; Bevilacqua, C.; Martin, P.; Philippe, C.; Walker, F.; Bado, A.; et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut 2012, 62, 1787–1794. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Shen, F.; Cao, H.-X.; Ding, W.-J.; Chen, Y.-W.; Fan, J.-G. Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals With Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Philips, C.A.; Pande, A.; Shasthry, S.M.; Jamwal, K.D.; Khillan, V.; Chandel, S.S.; Kumar, G.; Sharma, M.K.; Maiwall, R.; Jindal, A.; et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin. Gastroenterol. Hepatol. 2016, 15, 600–602. [Google Scholar] [CrossRef]
- Song, S.J.; Lauber, C.; Costello, E.K.; Lozupone, C.A.; Humphrey, G.; Berg-Lyons, D.; Caporaso, J.G.; Knights, D.; Clemente, J.C.; Nakielny, S.; et al. Cohabiting family members share microbiota with one another and with their dogs. eLife 2013, 2, e00458. [Google Scholar] [CrossRef]
- Xue, L.; Deng, Z.; Luo, W.; He, X.; Chen, Y. Effect of Fecal Microbiota Transplantation on Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Front. Cell. Infect. Microbiol. 2022, 12, 759306. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Ng, C.H.; Phang, P.H.; Chan, K.E.; Chin, Y.H.; Fu, C.E.; Zeng, R.W.; Xiao, J.; Tan, D.J.H.; Quek, J.; et al. Comparative Burden of Metabolic Dysfunction in Lean NAFLD vs Non-lean NAFLD-A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, S1542–S3565, 00669-3. [Google Scholar] [CrossRef] [PubMed]
| Patients | Type of Study | Probiotics Used | Outcomes | Ref |
|---|---|---|---|---|
| 48 children with NAFLD | Randomized clinical trials (RCT) | VSL#3 supplementation for 4 months vs. placebo | NAFLD reversal | [57] |
| 64 children with obesity and NAFLD | RCT (triple blind) | L. acidophilus, B. lactis, B. bifidum, L. rhamnosus | ↓ALT, AST, mean cholesterol, LDL-C, and TG in the probiotic-administered group | [58] |
| 38 patients (16 with NASH diagnosis): 7 treated with Probiotics, 9 with the standard of care vs. 22 healthy controls | RCT | Lactobacillus ssp. and Bifidobacterium bifidum | In NASH patients there was gut microbiota modulation: ↓Faecalibacterium ↓Anaerosporobacter ↑Parabacteroide ↑Allisonella | [59] |
| 48 T2DM NAFLD patients | RCT | “Symbiter Omega” probiotic biomass vs. placebo | ↓Fatty acids, ↓serum gamma-glutamyl transpeptidase ↓TG ↓TC | [60] |
| 58 patients with NAFLD and T2DM: 30 treated with probiotics vs. 28 receiving a placebo | RCT | biomass of 14 probiotic bacterial genera | In NAFLD patients: ↓Liver fat deposition ↓aminotransferase ↓TNF-α and IL-6 | [51] |
| 200 patients with NAFLD randomized to control group (standard of care treatment) and add-on treatments groups A, B, and C. | RCT | Bifidobacterium, Lactobacillus, Enterococcus, Bacillus subtilis, and Enterococcus | Amelioration of fatty liver deposition, ↓ALT, AST and TNF-α ↑HMW-APN | [61] |
| 75 patients with NASH under a low-fat/low-calorie diet | RCT | Lactobacilli, Bifidobacteria, Streptococcus thermophilus | Gut microbiota modulation towards “healthy” one, ↓BMI, ↓cholesterol in the probiotic-treated group | [62] |
| 50 patients (42 NAFLD) were randomized to probiotic or placebo | RCT | L. casei, L. acidophilus, L. rhamnosus, L. bulgaricus, B. breve, B. longum, S. thermophilus | ↓glycemic, inflammatory markers, Insulin, insulin resistance in NAFLD patients | [63] |
| 30 patients with NAFLD | RCT | L. bulgaricus and S. thermophilus | ↓ALT, AST, GGT in the probiotics group | [56] |
| 72 patients with NAFLD | RCT | Probiotic yogurt | ↓ALT, AST, TC, LDL-C | [64] |
| Study Type | Patients | Outcomes | RCT Number |
|---|---|---|---|
| FMT via nasojejunal tube | Diabetes and NAFLD | Improved HOMA index | NCT02469272 |
| FMT, pilot study | NAFLD and NASH | Improved degree of liver steatosis as assessed by MRI | NCT02469272 |
| FMT via duodenal infusion | NAFLD and NASH | Efficacy in NASH treatment vs. NAFLD | NCT03803540 |
| FMT via duodenal infusion | NAFLD and NASH | Reduction of hepatic venous gradient pressure | NCT02721264 |
| FMT vs. standard treatment (RCT) | Liver cirrhosis derived from NASH | Safety (e.g., number of adverse events, complications rate) | NCT02868164 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abenavoli, L.; Maurizi, V.; Rinninella, E.; Tack, J.; Di Berardino, A.; Santori, P.; Rasetti, C.; Procopio, A.C.; Boccuto, L.; Scarpellini, E. Fecal Microbiota Transplantation in NAFLD Treatment. Medicina 2022, 58, 1559. https://doi.org/10.3390/medicina58111559
Abenavoli L, Maurizi V, Rinninella E, Tack J, Di Berardino A, Santori P, Rasetti C, Procopio AC, Boccuto L, Scarpellini E. Fecal Microbiota Transplantation in NAFLD Treatment. Medicina. 2022; 58(11):1559. https://doi.org/10.3390/medicina58111559
Chicago/Turabian StyleAbenavoli, Ludovico, Valentina Maurizi, Emanuele Rinninella, Jan Tack, Arianna Di Berardino, Pierangelo Santori, Carlo Rasetti, Anna Caterina Procopio, Luigi Boccuto, and Emidio Scarpellini. 2022. "Fecal Microbiota Transplantation in NAFLD Treatment" Medicina 58, no. 11: 1559. https://doi.org/10.3390/medicina58111559
APA StyleAbenavoli, L., Maurizi, V., Rinninella, E., Tack, J., Di Berardino, A., Santori, P., Rasetti, C., Procopio, A. C., Boccuto, L., & Scarpellini, E. (2022). Fecal Microbiota Transplantation in NAFLD Treatment. Medicina, 58(11), 1559. https://doi.org/10.3390/medicina58111559










