Comparison of Clinical Characteristics and Predictors of Mortality between Direct and Indirect ARDS
Abstract
:1. Introduction
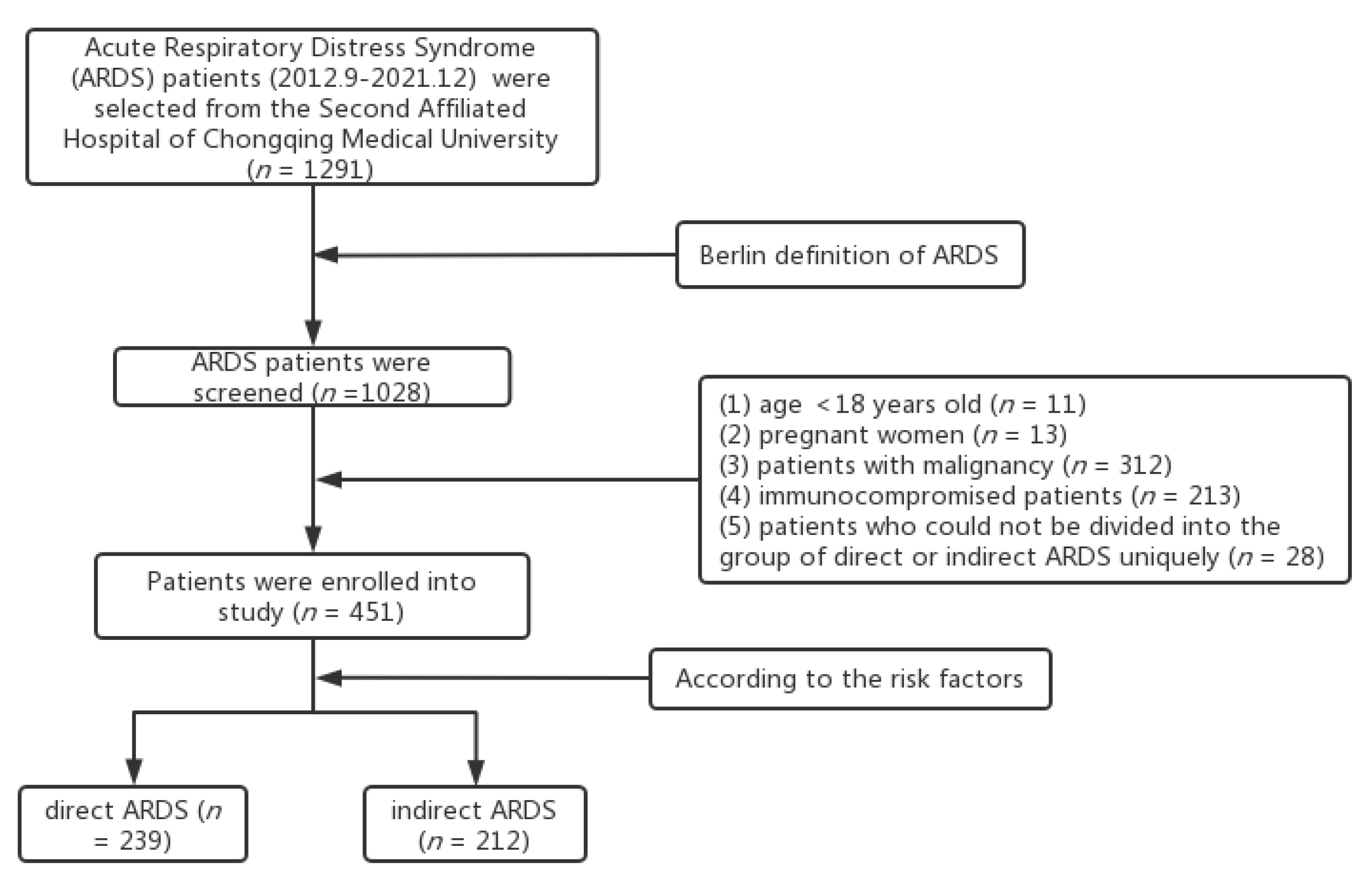
2. Materials and Methods
2.1. Study design
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Direct and Indirect ARDS
3.2. Clinical Characteristics of Survivors and Non-Survivors
3.3. Independent Predictors of 28-Day Mortality of Direct and Indirect ARDS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Moss, M.; Huang, D.T.; Brower, R.G. Early neuromuscular blockade in the acute respiratory distress syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar] [PubMed]
- Villar, J.; Blanco, J.; Añón, J.M.; Santos-Bouza, A.; Blanch, L.; Ambrós, A.; Gandía, F.; Carriedo, D.; Mosteiro, F.; Basaldúa, S.; et al. The ALIEN study: Incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011, 37, 1932–1941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [PubMed] [Green Version]
- Cartotto, R.; Li, Z.; Hanna, S.; Spano, S.; Wood, D.; Chung, K.; Camacho, F. The Acute Respiratory Distress Syndrome (ARDS) in mechanically ventilated burn patients: An analysis of risk factors, clinical features, and outcomes using the Berlin ARDS definition. Burns 2016, 42, 1423–1432. [Google Scholar] [CrossRef]
- Kallet, R.H.; Lipnick, M.S.; Zhuo, H.; Pangilinan, L.P.; Gomez, A. Characteristics of Nonpulmonary Organ Dysfunction at Onset of ARDS Based on the Berlin Definition. Respir. Care 2019, 64, 493–501. [Google Scholar] [CrossRef]
- Yu, S.; Christiani, D.C.; Thompson, B.T.; Bajwa, E.K.; Gong, M.N. Role of diabetes in the development of acute respiratory distress syndrome. Crit. Care Med. 2013, 41, 2720–2732. [Google Scholar] [CrossRef]
- Kor, D.J.; Warner, D.O.; Alsara, A.; Fernández-Pérez, E.R.; Malinchoc, M.; Kashyap, R.; Li, G.; Gajic, O. Derivation and diagnostic accuracy of the surgical lung injury prediction model. J. Am. Soc. Anesthesiol. 2011, 115, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Singla, A.; Turner, P.; Pendurthi, M.K. Effect of type II diabetes mellitus on outcomes in patients with acute respiratory distress syndrome. J. Crit. Care 2014, 29, 66–69. [Google Scholar] [CrossRef]
- Callister, M.E.; Evans, T.W. Pulmonary versus extrapulmonary acute respiratory distress syndrome: Different diseases or just a useful concept? Curr. Opin. Crit. Care 2002, 8, 21–25. [Google Scholar] [CrossRef]
- Bernard, G.R.; Artigas, A.; Brigham, K.L.; Carlet, J.; Falke, K.; Hudson, L.; Lamy, M.; Legall, J.R.; Morris, A.; Spragg, R. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am. J. Respir. Crit. Care Med. 1994, 149, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.A.; Fan, E.; Ferguson, N.D. Precision Medicine and Heterogeneity of Treatment Effect in Therapies for ARDS. Chest 2021, 160, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Capelozzi, V.L.; Allen, T.C.; Beasley, M.B.; Cagle, P.T.; Guinee, D.; Hariri, L.P.; Husain, A.N.; Jain, D.; Lantuejoul, S.; Larsen, B.T.; et al. Molecular and immune biomarkers in acute respiratory distress syndrome: A perspective from members of the pulmonary pathology society. Arch. Pathol. Lab. Med. 2017, 141, 1719–1727. [Google Scholar] [CrossRef] [Green Version]
- Shaver, C.M.; Bastarache, J.A. Clinical and biological heterogeneity in acute respiratory distress syndrome: Direct versus indirect lung injury. Clin. Chest Med. 2014, 35, 639–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoelz, C.; Negri, E.M.; Lichtenfels, A.J.; Conceição, G.M.; Barbas, C.S.; Saldiva, P.H.; Capelozzi, V.L. Morphometric differences in pulmonary lesions in primary and secondary ARDS. A preliminary study in autopsies. Pathol. Res. Pract. 2001, 197, 521–530. [Google Scholar]
- Calfee, C.S.; Janz, D.R.; Bernard, G.R.; May, A.K.; Kangelaris, K.N.; Matthay, M.A.; Ware, L.B. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015, 147, 1539–1548. [Google Scholar] [CrossRef] [Green Version]
- Sheu, C.-C.; Gong, M.N.; Zhai, R.; Chen, F.; Bajwa, E.K.; Clardy, P.F.; Gallagher, D.C.; Thompson, B.T.; Christiani, D.C. Clinical characteristics and outcomes of sepsis-related vs non-sepsis-related ARDS. Chest 2010, 138, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Shaver, C.M.; Zhao, Z.; Koyama, T.; Calfee, C.S.; Bastarache, J.A.; Ware, L.B. Clinical Predictors of Hospital Mortality Differ Between Direct and Indirect ARDS. Chest 2017, 151, 755–763. [Google Scholar] [CrossRef] [Green Version]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Cooke, C.R.; Kahn, J.M.; Caldwell, E.; Okamoto, V.N.; Heckbert, S.R.; Hudson, L.D.; Rubenfeld, G.D. Predictors of hospital mortality in a population-based cohort of patients with acute lung injury. Crit. Care Med. 2008, 36, 1412–1420. [Google Scholar] [CrossRef]
- Antonini, J.M.; Roberts, J.R.; Clarke, R.W.; Yang, H.M.; Barger, M.W.; Ma, J.Y.; Weissman, D.N. Effect of age on respiratory defense mechanisms: Pulmonary bacterial clearance in Fischer 344 rats after intratracheal instillation of Listeria monocytogenes. Chest 2001, 120, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Redente, E.F.; Jacobsen, K.M.; Solomon, J.J.; Lara, A.R.; Faubel, S.; Keith, R.C.; Henson, P.M.; Downey, G.P.; Riches, D.W. Age and sex dimorphisms contribute to the severity of bleomycin-induced lung injury and fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2011, 301, L510–L518. [Google Scholar] [CrossRef] [PubMed]
- Schouten, L.R.; Schultz, M.J.; van Kaam, A.H.; Juffermans, N.P.; Bos, A.P.; Wösten-van Asperen, R.M. Association between Maturation and Aging and Pulmonary Responses in Animal Models of Lung Injury: A Systematic Review. Anesthesiology 2015, 123, 389–408. [Google Scholar] [CrossRef]
- Bodas, M.; Min, T.; Vij, N. Early-age-related changes in proteostasis augment immunopathogenesis of sepsis and acute lung injury. PLoS ONE 2010, 5, e15480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchyta, M.R. The changing face of organ failure in ARDS. Chest 2003, 124, 1871–1879. [Google Scholar] [CrossRef] [Green Version]
- Martin, E.L.; Souza, D.G.; Fagundes, C.T.; Amaral, F.A.; Assenzio, B.; Puntorieri, V.; Del Sorbo, L.; Fanelli, V.; Bosco, M.; Delsedime, L.; et al. Phosphoinositide-3 kinase {gamma} activity contributes to sepsis and organ damage by altering neutrophil recruitment. Am. J. Respir. Crit. Care Med. 2010, 182, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Ye, Q.; Wan, Q.; Zhou, J. Mortality predictors in recipients developing acute respiratory distress syndrome due to pneumonia after kidney transplantation. Ren. Fail. 2016, 38, 1082–1088. [Google Scholar] [CrossRef] [Green Version]
- Seeley, E.J. Updates in the management of acute lung injury: A focus on the overlap between AKI and ARDS. Adv. Chronic Kidney Dis. 2013, 20, 14–20. [Google Scholar] [CrossRef]
- Park, B.D.; Faubel, S. Acute Kidney Injury and Acute Respiratory Distress Syndrome. Crit. Care Clin. 2021, 37, 835–849. [Google Scholar] [CrossRef]
- Betjes, M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Andres-Hernando, A.; Dursun, B.; Altmann, C.; Ahuja, N.; He, Z.; Bhargava, R.; Edelstein, C.E.; Jani, A.; Hoke, T.S.; Klein, C.; et al. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol. Dial. Transplant. 2012, 27, 4339–4347. [Google Scholar] [CrossRef] [PubMed]
- Rabb, H.; Wang, Z.; Nemoto, T.; Hotchkiss, J.; Yokota, N.; Soleimani, M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003, 63, 600–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baranovskii, D.S.; Klabukov, I.D.; Krasilnikova, O.A.; Nikogosov, D.A.; Polekhina, N.V.; Baranovskaia, D.R.; Laberko, L.A. Prolonged prothrombin time as an early prognostic indicator of severe acute respiratory distress syndrome in patients with COVID-19 related pneumonia. Curr. Med. Res. Opin. 2021, 37, 21–25. [Google Scholar] [CrossRef]
- Frantzeskaki, F.; Armaganidis, A.; Orfanos, S.E. Immunothrombosis in Acute Respiratory Distress Syndrome: Cross Talks between Inflammation and Coagulation. Respiration 2017, 93, 212–225. [Google Scholar] [CrossRef]
- Ware, L.B.; Fang, X.; Matthay, M.A. Protein C and thrombomodulin in human acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L514–L521. [Google Scholar] [CrossRef] [Green Version]
- Palanidurai, S. P/FP ratio: Incorporation of PEEP into the PaO2/FiO2 ratio for prognostication and classification of acute respiratory distress syndrome. Ann. Intensive Care 2021, 11, 124. [Google Scholar] [CrossRef]
- Bajwa, E.K.; Khan, U.A.; Januzzi, J.L.; Gong, M.N.; Thompson, B.T.; Christiani, D.C. Plasma C-reactive protein levels are associated with improved outcome in ARDS. Chest 2009, 136, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Buchta, R.; Fridkin, M.; Pontet, M.; Contessi, E.; Scaggiante, B.; Romeo, D. Modulation of human neutrophil function by C-reactive protein. Eur. J. Biochem. 1987, 163, 141–146. [Google Scholar] [CrossRef]
- Heuertz, R.M.; Tricomi, S.M.; Ezekiel, U.R.; Webster, R.O. C-reactive protein inhibits chemotactic peptide-induced p38 mitogen-activated protein kinase activity and human neutrophil movement. J. Biol. Chem. 1999, 274, 17968–17974. [Google Scholar] [CrossRef] [Green Version]
- Heuertz, R.M.; Piquette, C.A.; Webster, R.O. Rabbits with elevated serum C-reactive protein exhibit diminished neutrophil infiltration and vascular permeability in C5a-induced alveolitis. Am. J. Pathol. 1993, 142, 319–328. [Google Scholar] [PubMed]
| Characteristic | Total ARDS (n = 451) | Direct ARDS (n = 239) | Indirect ARDS (n = 212) | p-Value |
|---|---|---|---|---|
| Male, n (%) | 300 (66.5) | 172 (72.0) | 128 (60.4) | 0.009 |
| Age (years) | 63.0 (47.0–75.0) | 70.0 (59.0–78.0) | 50.0 (41.3–66.0) | < 0.001 |
| Alcohol, n (%) | 132 (29.3) | 64 (26.8) | 68 (32.1) | 0.217 |
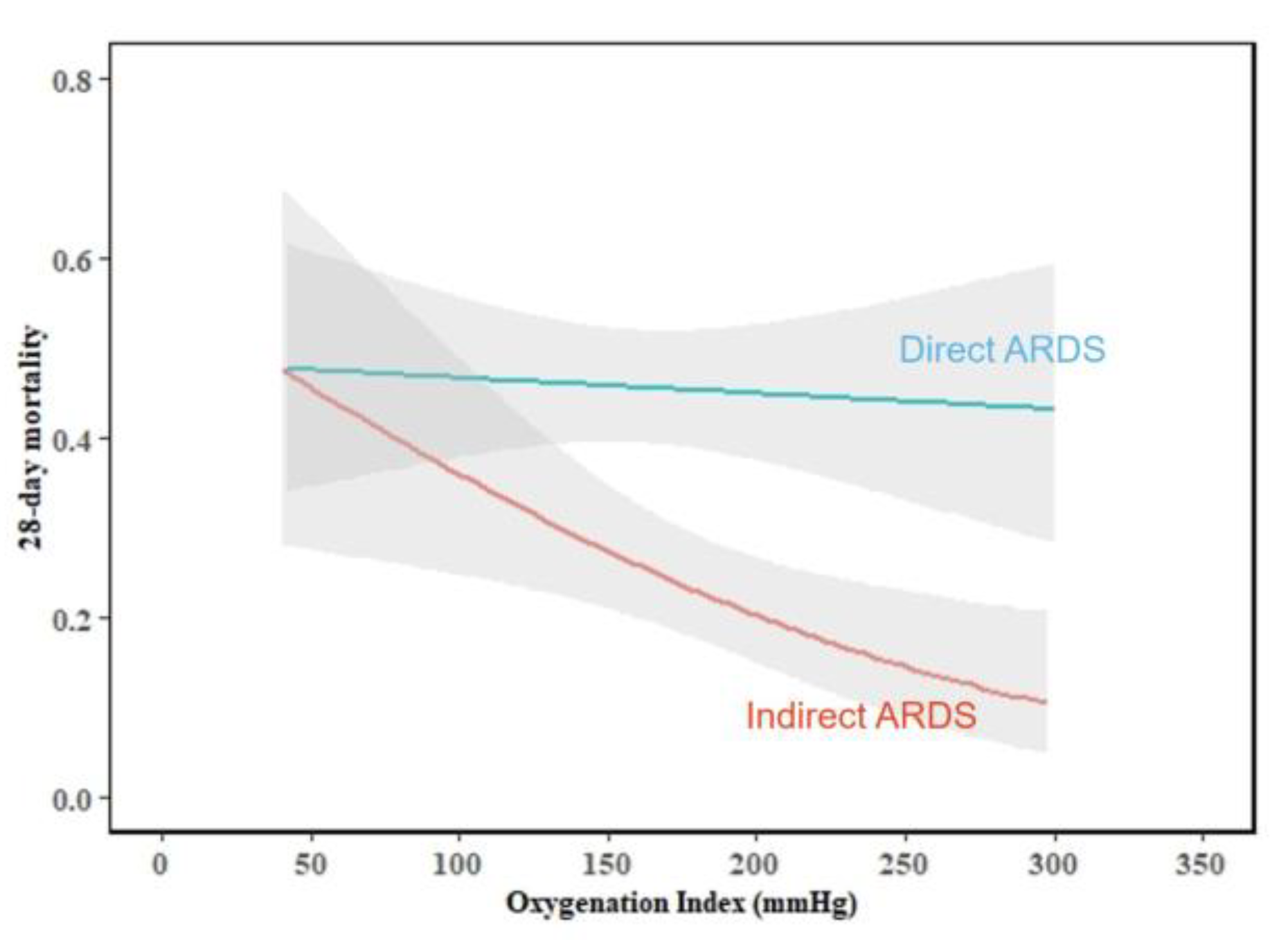
| Oxygenation index (mmHg) | 171.0 (127.5–215.0) | 154.1 (118.2–203.0) | 184.5 (141.8–227.8) | < 0.001 |
| Comorbidity, n (%) | ||||
| Diabetes, n (%) | 101 (22.4) | 60 (25.1) | 41 (19.3) | 0.143 |
| Hypertension, n (%) | 146 (32.4) | 101 (42.3) | 45 (21.2) | < 0.001 |
| Coronary heart disease, n (%) | 57 (12.6) | 48 (20.1) | 9 (4.2) | < 0.001 |
| COPD, n (%) | 50 (11.1) | 47 (19.7) | 3 (1.4) | < 0.001 |
| MODS, n (%) | 107 (23.7) | 62 (25.9) | 45 (21.2) | 0.240 |
| aCCI | 4.0 (2.0–5.0) | 5.0 (3.0–6.0) | 2.0 (1.0–4.0) | < 0.001 |
| Laboratory findings | ||||
| Platelet (×109/L) | 165.0 (109.0–228.0) | 173.0 (109.0–252.0) | 158.5 (108.3–215.0) | 0.115 |
| NLR | 13.0 (7.7–21.7) | 12.5 (6.8–21.0) | 14.0 (8.4–22.2) | 0.235 |
| CRP (ng/mL) | 135.6 (55.4–200.0) | 102.2 (34.7–176.3) | 182.4 (97.6–200.0) | < 0.001 |
| Procalcitonin (ng/mL) | 1.5 (0.4–8.4) | 0.9 (0.2–6.5) | 2.6 (0.6–12.7) | < 0.001 |
| Albumin (g/L) | 30.2 (25.8–34.0) | 30.2 (25.1–33.6) | 30.4 (26.8–34.4) | 0.113 |
| Blood glucose (mmol/L) | 8.7 (6.6–12.3) | 8.1 (6.3–11.0) | 9.8 (7.4–14.0) | <0.001 |
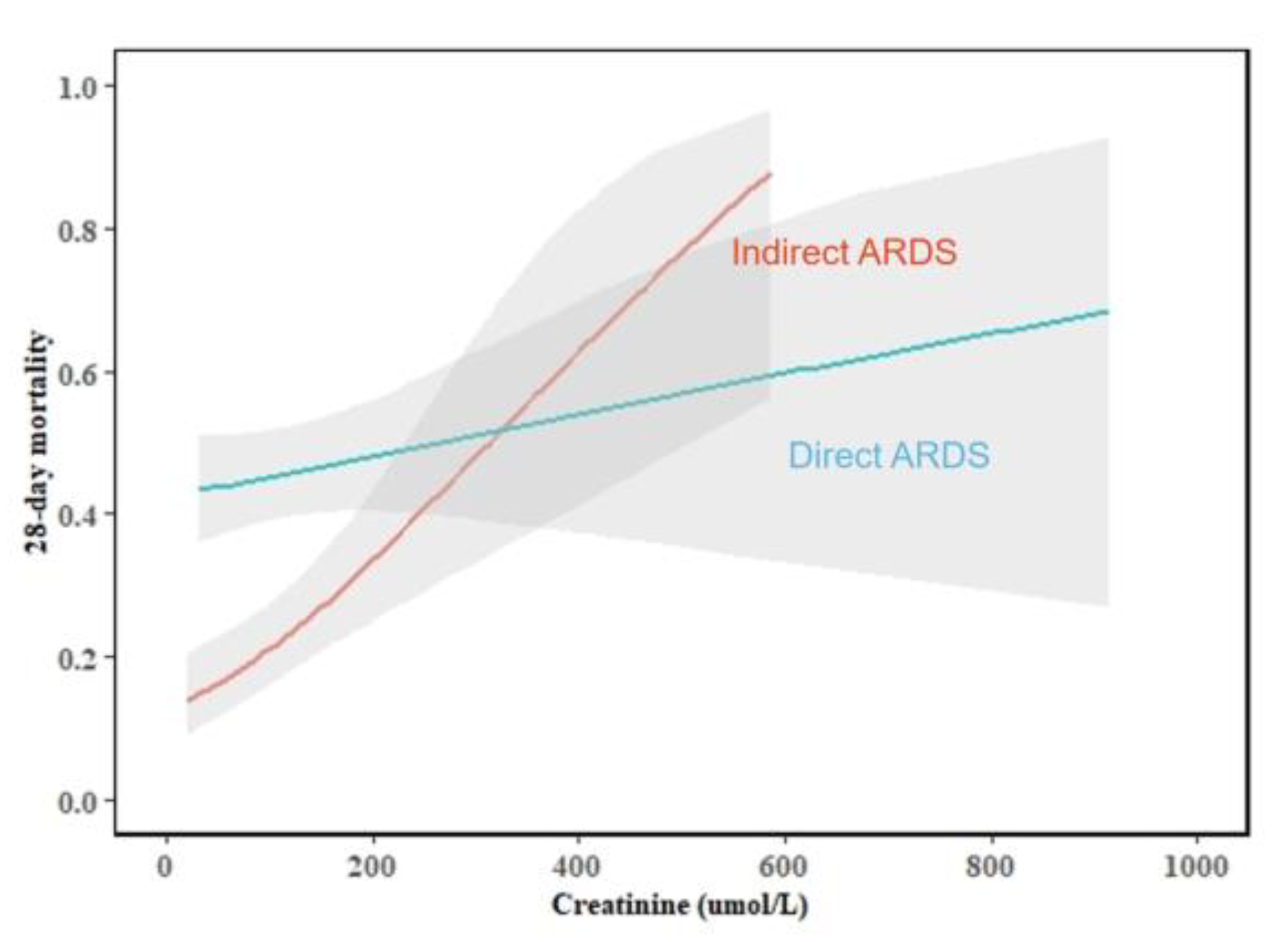
| Creatinine (umol/L) | 76.1 (52.4–121.6) | 79.1 (56.6–120.8) | 73.7 (50.3–122.2) | 0.147 |
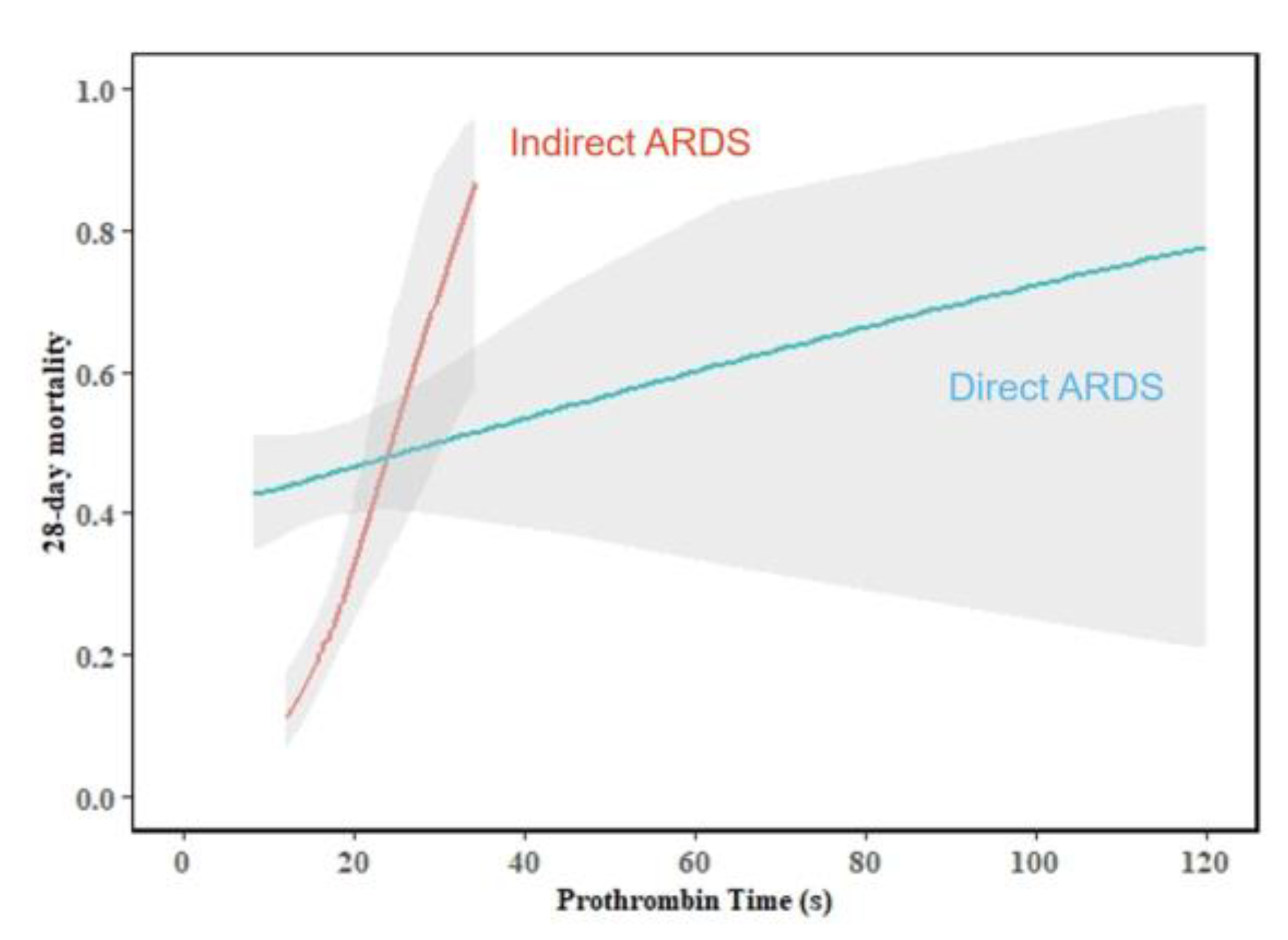
| PT (s) | 15.1 (14.0–17.0) | 14.8 (13.8–16.4) | 15.5 (14.1–17.4) | 0.003 |
| APTT (s) | 42.1 (36.9–47.7) | 41.7 (36.5–47.2) | 42.2 (37.2–48.1) | 0.221 |
| Scores | ||||
| SOFA | 6.0 (4.0–8.0) | 6.0 (4.0–8.0) | 6.0 (4.0–9.0) | 0.360 |
| APACHEⅡ | 18.0 (14.0–23.0) | 19.0 (15.0–24.0) | 16.0 (12.0–21.0) | 0.001 |
| Therapy, n (%) | ||||
| IMV, n (%) | 152 (33.7) | 81 (33.9) | 71 (33.5) | 0.928 |
| Glucocorticoid, n (%) | 181 (45.1) | 147 (61.5) | 34 (16.0) | < 0.001 |
| Vasopressor, n (%) | 185 (41.0) | 116 (48.5) | 69 (32.5) | 0.001 |
| Rehabilitation, n (%) | 298 (66.1) | 140 (58.6) | 158 (74.5) | < 0.001 |
| Outcome | ||||
| 28-day mortality, n (%) | 158 (35.0) | 109 (45.6) | 49 (23.1) | < 0.001 |
| Hospital LOS, n (d) | 15 (7–24) | 14 (6–22) | 18 (9–28) | < 0.001 |
| Characteristic | Direct ARDS (n = 239) | p-Value | Indirect ARDS (n = 212) | p-Value | ||
|---|---|---|---|---|---|---|
| Survivors (n = 130, 54.4%) | Non-Survivors (n = 109, 45.6%) | Survivors (n = 163, 76.9%) | Non-Survivors (n = 49, 23.1%) | |||
| Male, n (%) | 88 (67.7) | 84 (77.1) | 0.108 | 97 (59.5) | 31 (63.3) | 0.637 |
| Age (years) | 66.0 (56.0–75.0) | 74.0 (64.5–82.0) | < 0.001 | 50.0 (41.0–63.0) | 52.0 (41.5–78.5) | 0.096 |
| Alcohol, n (%) | 31 (23.8) | 33 (30.3) | 0.264 | 55 (33.7) | 13 (26.5) | 0.343 |
| Oxygenation index (mmHg) | 160.8 (121.4–195.3) | 143.9 (111.0–210.3) | 0.593 | 188.0 (152.0–230.0) | 174.0 (117.0–213.0) | 0.024 |
| Comorbidity, n (%) | ||||||
| Diabetes, n (%) | 35 (26.9) | 25 (22.9) | 0.479 | 35 (21.5) | 6 (12.2) | 0.152 |
| Hypertension, n (%) | 59 (45.4) | 42 (38.5) | 0.285 | 35 (21.5) | 10 (20.4) | 0.873 |
| Coronary heart disease, n (%) | 22 (16.9) | 26 (23.9) | 0.183 | 5 (3.1) | 4 (8.2) | 0.121 |
| COPD, n (%) | 27 (20.8) | 20 (18.3) | 0.639 | 1 (0.6) | 2 (4.1) | 0.072 |
| MODS, n (%) | 24 (18.5) | 38 (34.9) | 0.004 | 21 (12.9) | 24 (49.0) | < 0.001 |
| aCCI | 4.0 (3.0–5.0) | 5.0 (4.0–6.0) | 0.001 | 2.0 (1.0–4.0) | 3.0 (1.0–5.0) | 0.014 |
| Laboratory findings | ||||||
| Platelet (×109/L) | 175.0 (123.0–248.8) | 167.0 (98.0–256.5) | 0.579 | 164.0 (115.0–216.0) | 133.0 (70.5–208.5) | 0.027 |
| NLR | 12.8 (7.6–21.1) | 12.0 (6.1–22.0) | 0.333 | 13.7 (8.1–21.1) | 15.2 (8.8–28.2) | 0.214 |
| CRP (ng/Ml) | 95.7 (34.4–179.0) | 116.5 (37.0–172.9) | 0.706 | 200.0 (116.4–200.0) | 148.7 (53.8–200.0) | 0.006 |
| Procalcitonin (ng/mL) | 0.7 (0.2–6.4) | 1.4 (0.2–7.0) | 0.575 | 1.8 (0.5–7.1) | 6.9 (1.1–20.4) | 0.007 |
| Albumin(g/L) | 30.6 (26.1–34.1) | 28.6 (24.3–33.5) | 0.102 | 30.5 (27.9–35.0) | 28.6 (24.3–32.9) | 0.015 |
| Blood glucose (mmol/L) | 8.1 (6.4–11.0) | 8.2 (6.0–11.3) | 0.697 | 9.7 (7.4–13.8) | 10.1 (7.8–14.6) | 0.355 |
| Creatinine (umol/L) | 70.1 (55.2–100.8) | 91.1 (60.6–136.9) | 0.006 | 64.0 (47.8–98.5) | 127.4 (74.6–224.3) | < 0.001 |
| PT (s) | 14.6 (13.5–15.8) | 15.3 (14.1–17.5) | 0.002 | 15.3 (14.1–16.6) | 17.6 (14.9–21.1) | < 0.001 |
| APTT (s) | 41.7 (36.1–46.9) | 42.1 (37.0–48.3) | 0.508 | 41.4 (36.7–47.1) | 46.2 (37.5–67.7) | 0.005 |
| Scores | ||||||
| SOFA | 5.0 (4.0–7.0) | 7.0 (4.0–9.0) | < 0.001 | 5.0 (4.0,8.0) | 10.0 (6.5,12.0) | < 0.001 |
| APACHEⅡ | 17.5 (14.0–22.0) | 21.0 (16.0–24.5) | 0.003 | 15.0 (11.0,19.0) | 25.0 (17.5,30.5) | < 0.001 |
| Therapy, n (%) | ||||||
| IMV, n (%) | 35 (26.9) | 46 (42.2) | 0.013 | 37 (22.7) | 34 (69.4) | < 0.001 |
| Glucocorticoid, n (%) | 73 (56.2) | 74 (67.9) | 0.063 | 24 (14.7) | 10 (20.4) | 0.342 |
| Vasopressor, n (%) | 33 (25.4) | 83 (76.1) | < 0.001 | 29 (17.8) | 40 (81.6) | < 0.001 |
| Rehabilitation, n (%) | 79 (60.8) | 61 (56.0) | 0.453 | 121 (74.2) | 37 (75.5) | 0.857 |
| Characteristic | Total ARDS (n = 451) | Direct ARDS (n = 239) | Indirect ARDS (n = 212) | p-Value * | |||
|---|---|---|---|---|---|---|---|
| Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | ||
| Age (years) | 1.034 (1.021–1.048) | < 0.001 | 1.032 (1.012–1.053) | 0.002 | 1.034 (1.010–1.059) | 0.005 | |
| MODS | 2.972 (1.802–4.902) | < 0.001 | 2.059 (1.092–3.879) | 0.026 | 4.370 (1.755–10.882) | 0.002 | |
| Platelet (×109/L) | 1.000 (0.998–1.002) | 0.949 | 1.000 (0.998–1.002) | 0.838 | 1.000 (0.996–1.004) | 0.999 | |
| CRP (ng/mL) | 0.997 (0.994–1.000) | 0.054 | 0.999 (0.996–1.003) | 0.791 | 0.996 (0.991–1.002) | 0.193 | |
| Procalcitonin (ng/mL) | 1.000 (0.997–1.004) | 0.843 | 1.003 (0.996–1.010) | 0.396 | 0.995 (0.986–1.004) | 0.235 | |
| Albumin(g/L) | 0.970 (0.936–1.005) | 0.089 | 0.981 (0.938–1.027) | 0.413 | 0.976 (0.916–1.040) | 0.456 | |
| Creatinine (umol/L) | 1.002 (1.000–1.004) | 0.049 | 1.001 (0.998–1.003) | 0.637 | 1.005 (1.001–1.010) | 0.017 | 0.001 |
| PT (s) | 1.022 (0.992–1.053) | 0.148 | 1.010 (0.983–1.038) | 0.460 | 1.161 (1.043–1.292) | 0.006 | < 0.001 |
| APTT (s) | 1.003 (0.994–1.011) | 0.510 | 1.005 (0.990–1.020) | 0.555 | 0.994 (0.980–1.008) | 0.388 | |
| Oxygenation index (mmHg) | 0.996 (0.992–0.999) | 0.023 | 0.999 (0.995–1.004) | 0.810 | 0.991 (0.984–0.998) | 0.010 | < 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, W.; Tang, R.; Zhao, Y.; Peng, J.; Wang, D. Comparison of Clinical Characteristics and Predictors of Mortality between Direct and Indirect ARDS. Medicina 2022, 58, 1563. https://doi.org/10.3390/medicina58111563
Tang W, Tang R, Zhao Y, Peng J, Wang D. Comparison of Clinical Characteristics and Predictors of Mortality between Direct and Indirect ARDS. Medicina. 2022; 58(11):1563. https://doi.org/10.3390/medicina58111563
Chicago/Turabian StyleTang, Wen, Rui Tang, Yan Zhao, Junnan Peng, and Daoxin Wang. 2022. "Comparison of Clinical Characteristics and Predictors of Mortality between Direct and Indirect ARDS" Medicina 58, no. 11: 1563. https://doi.org/10.3390/medicina58111563




