Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study
Abstract
:1. Introduction
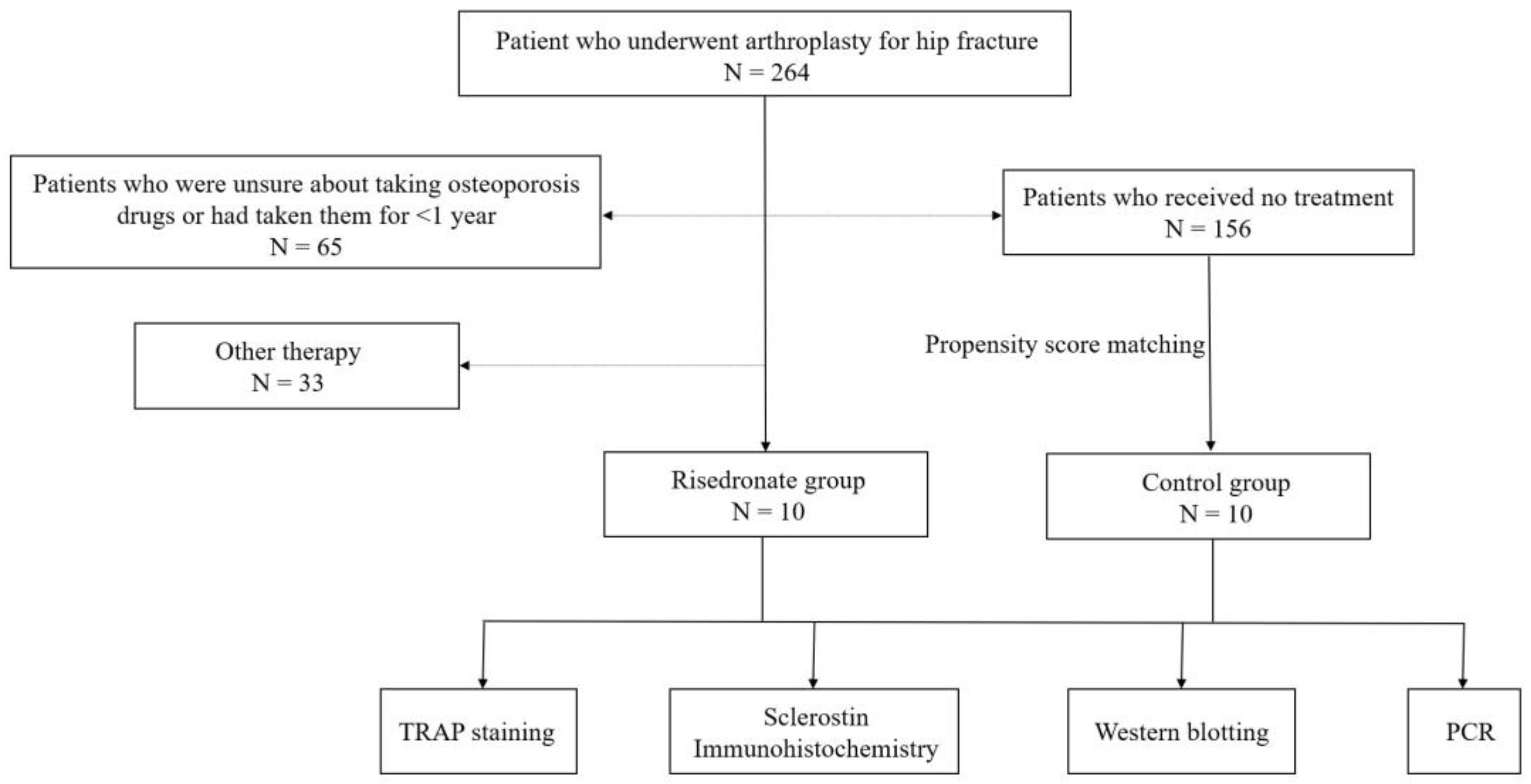
2. Materials and Methods
2.1. Specimen Collection
2.2. Histological Analysis of Decalcified Bone Tissue
2.3. TRAP Staining
2.4. Sclerostin Immunohistochemistry
2.5. Western Blotting
2.6. Quantitative Real-Time Polymerase Chain Reaction (PCR)
2.7. Statistical Analysis
3. Results
3.1. Specimen Collection
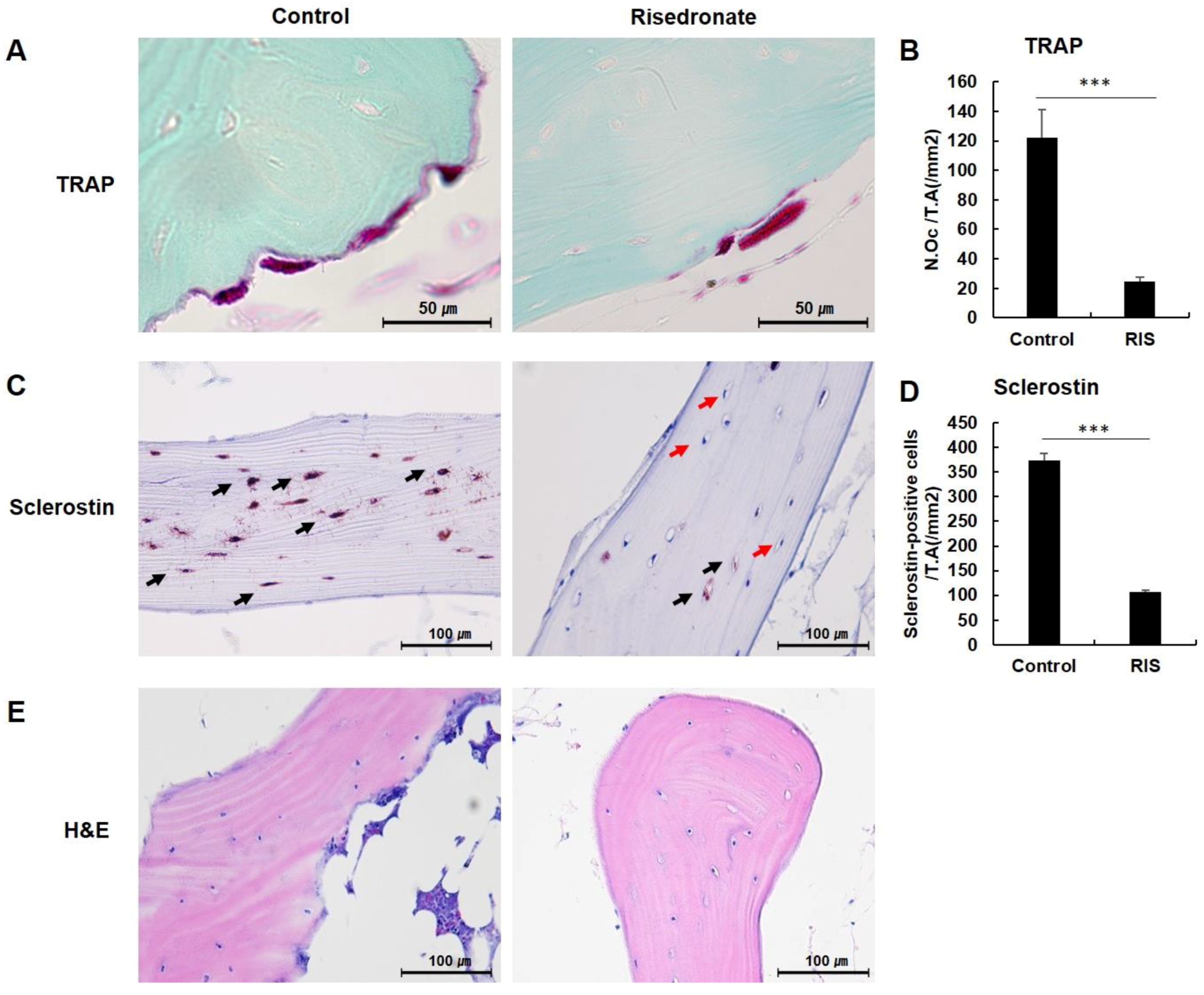
3.2. TRAP Staining
3.3. Sclerostin Immunohistochemistry
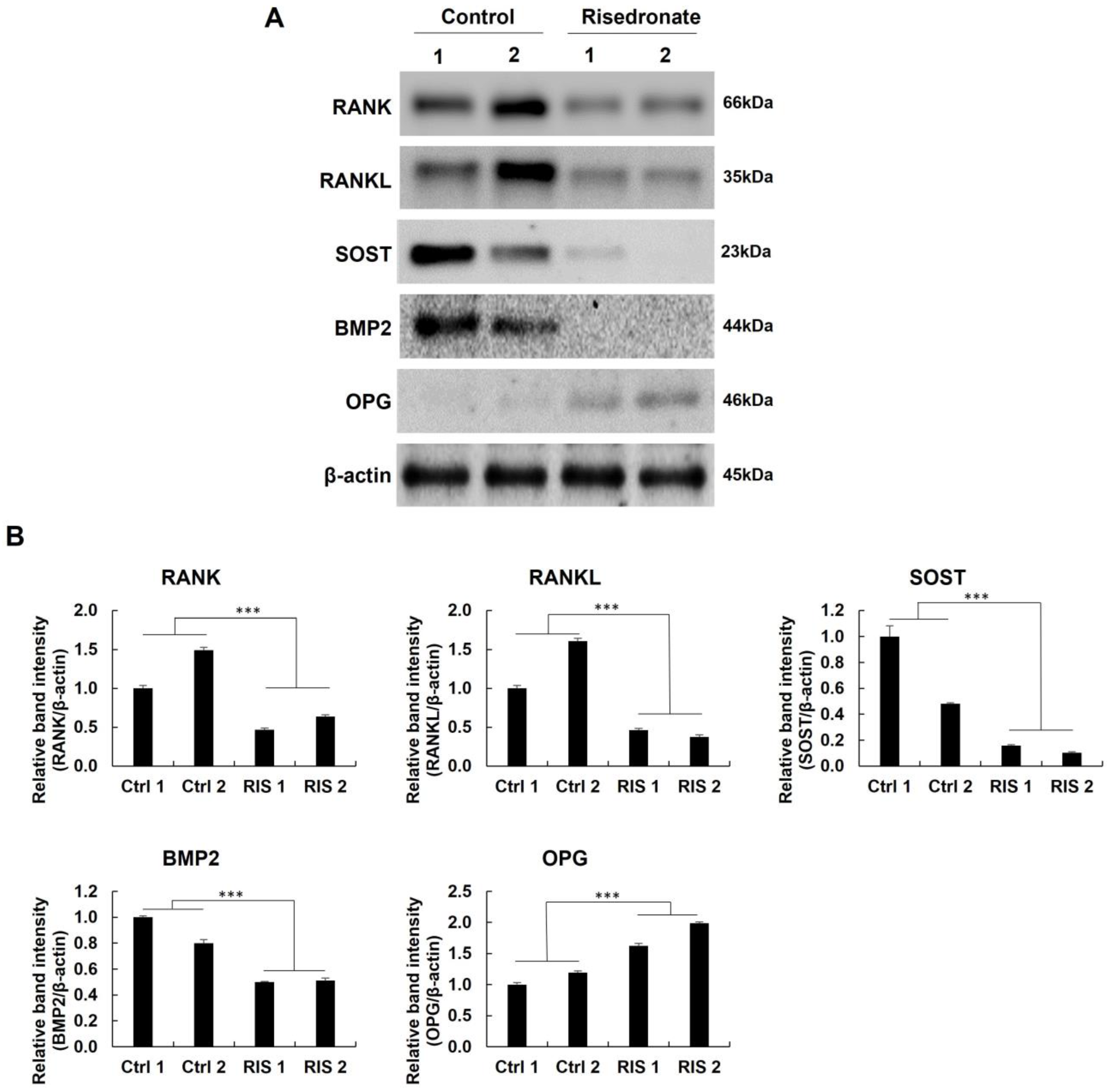
3.4. Western Blotting and Quantitative Real-Time PCR
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McClung, M.R.; O’Donoghue, M.L.; Papapoulos, S.E.; Bone, H.; Langdahl, B.; Saag, K.G.; Reid, I.R.; Kiel, D.P.; Cavallari, I.; Bonaca, M.P.; et al. Odanacatib for the treatment of postmenopausal osteoporosis: Results of the LOFT multicentre, randomised, double-blind, placebo-controlled trial and LOFT Extension study. Lancet Diabetes Endocrinol. 2019, 7, 899–911. [Google Scholar] [CrossRef]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, T.; Fukunaga, M.; Nakano, T.; Kishimoto, H.; Ito, M.; Hagino, H.; Sone, T.; Taguchi, A.; Tanaka, S.; Ohashi, M.; et al. Efficacy and safety of once-yearly zoledronic acid in Japanese patients with primary osteoporosis: Two-year results from a randomized placebo-controlled double-blind study (ZOledroNate treatment in Efficacy to osteoporosis; ZONE study). Osteoporos. Int. 2017, 28, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Boivin, G.Y.; Chavassieux, P.M.; Santora, A.C.; Yates, J.; Meunier, P.J. Alendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 2000, 27, 687–694. [Google Scholar] [CrossRef]
- Wells, G.A.; Hsieh, S.C.; Zheng, C.; Peterson, J.; Tugwell, P.; Liu, W. Risedronate for the primary and secondary prevention of osteoporotic fractures in postmenopausal women. Cochrane Database Syst. Rev. 2022, 5, Cd004523. [Google Scholar] [CrossRef]
- Deardorff, W.J.; Cenzer, I.; Nguyen, B.; Lee, S.J. Time to Benefit of Bisphosphonate Therapy for the Prevention of Fractures Among Postmenopausal Women With Osteoporosis: A Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R. Updates on mechanism of action and clinical efficacy of risedronate in osteoporosis. Clin. Cases Min. Bone Metab. 2014, 11, 208–214. [Google Scholar] [CrossRef]
- Dunn, C.J.; Goa, K.L. Risedronate: A review of its pharmacological properties and clinical use in resorptive bone disease. Drugs 2001, 61, 685–712. [Google Scholar] [CrossRef]
- Ke, C.H.; Li, H.Y.; Yang, D.; Ying, H.; Xu, J.; Wang, J.; Zhu, H.W.; Wang, L. Dynamic Effects of the Third Generation Bisphosphonate of Risedronate on Rat Osteoporotic Fractures for Clinical Usage Guidance. Orthop. Surg. 2021, 13, 2433–2441. [Google Scholar] [CrossRef]
- Yang, L. The efficiency of risedronate in reducing bone resorption after total hip arthroplasty: A meta-analysis of randomized control trials at a minimum of 6 months’ follow-up. J. Orthop. Surg. Res. 2018, 13, 88. [Google Scholar] [CrossRef]
- Viswanathan, M.; Reddy, S.; Berkman, N.; Cullen, K.; Middleton, J.C.; Nicholson, W.K.; Kahwati, L.C. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. In Screening to Prevent Osteoporotic Fractures: An Evidence Review for the U.S. Preventive Services Task Force; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2018. [Google Scholar]
- Watts, N.B. Fundamentals and pitfalls of bone densitometry using dual-energy X-ray absorptiometry (DXA). Osteoporos Int. 2004, 15, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, H.H. DXA in vivo BMD methodology: An erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone 2007, 41, 138–154. [Google Scholar] [CrossRef] [PubMed]
- Nakatoh, S. Effect of osteoporosis medication on changes in bone mineral density and bone turnover markers after 24-month administration of daily teriparatide: Comparison among minodronate, raloxifene, and eldecalcitol. J. Bone Miner. Metab. 2018, 36, 221–228. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Spiezia, F.; Tingart, M.; Maria, P.G.; Riccardo, G. Biomarkers as therapy monitoring for postmenopausal osteoporosis: A systematic review. J. Orthop. Surg. Res. 2021, 16, 318. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, K.K.; McGuigan, F.E.; Malmgren, L.; Gerdhem, P.; Johansson, H.; Kanis, J.A.; Akesson, K.E. Bone Turnover Marker Profiling and Fracture Risk in Older Women: Fracture Risk from Age 75 to 90. Calcif. Tissue Int. 2022, 111, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Gorter, E.A.; Reinders, C.R.; Krijnen, P.; Appelman-Dijkstra, N.M.; Schipper, I.B. Serum sclerostin levels in osteoporotic fracture patients. Eur. J. Trauma Emerg. Surg. 2022. [Google Scholar] [CrossRef]
- Ardawi, M.S.; Rouzi, A.A.; Al-Sibiani, S.A.; Al-Senani, N.S.; Qari, M.H.; Mousa, S.A. High serum sclerostin predicts the occurrence of osteoporotic fractures in postmenopausal women: The Center of Excellence for Osteoporosis Research Study. J. Bone Miner. Res. 2012, 27, 2592–2602. [Google Scholar] [CrossRef]
- Ozaki, Y.; Koide, M.; Furuya, Y.; Ninomiya, T.; Yasuda, H.; Nakamura, M.; Kobayashi, Y.; Takahashi, N.; Yoshinari, N.; Udagawa, N. Treatment of OPG-deficient mice with WP9QY, a RANKL-binding peptide, recovers alveolar bone loss by suppressing osteoclastogenesis and enhancing osteoblastogenesis. PLoS ONE 2017, 12, e0184904. [Google Scholar] [CrossRef] [Green Version]
- Inacio, M.C.; Chen, Y.; Paxton, E.W.; Namba, R.S.; Kurtz, S.M.; Cafri, G. Statistics in Brief: An Introduction to the Use of Propensity Scores. Clin. Orthop. Relat. Res. 2015, 473, 2722–2726. [Google Scholar] [CrossRef] [Green Version]
- Balemans, W.; Ebeling, M.; Patel, N.; Van Hul, E.; Olson, P.; Dioszegi, M.; Lacza, C.; Wuyts, W.; Van Den Ende, J.; Willems, P.; et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 2001, 10, 537–543. [Google Scholar] [CrossRef]
- Moester, M.J.; Papapoulos, S.E.; Löwik, C.W.; van Bezooijen, R.L. Sclerostin: Current knowledge and future perspectives. Calcif. Tissue Int. 2010, 87, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Winkler, D.G.; Sutherland, M.K.; Geoghegan, J.C.; Yu, C.; Hayes, T.; Skonier, J.E.; Shpektor, D.; Jonas, M.; Kovacevich, B.R.; Staehling-Hampton, K.; et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J. 2003, 22, 6267–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semënov, M.; Tamai, K.; He, X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J. Biol. Chem. 2005, 280, 26770–26775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Santana, S.; Díez-Pérez, A.; Olmos, J.M.; Nogués, X.; Sosa, M.; Díaz-Curiel, M.; Pérez-Castrillón, J.L.; Pérez-Cano, R.; Torrijos, A.; Jodar, E.; et al. Circulating sclerostin and estradiol levels are associated with inadequate response to bisphosphonates in postmenopausal women with osteoporosis. Maturitas 2015, 82, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Anastasilakis, A.D.; Bratengeier, C.; Woloszczuk, W.; Papatheodorou, A.; Terpos, E. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women–the six-month effect of risedronate and teriparatide. Osteoporos. Int. 2012, 23, 1171–1176. [Google Scholar] [CrossRef]
- Boltenstål, H.; Qureshi, A.R.; Behets, G.J.; Lindholm, B.; Stenvinkel, P.; D’Haese, P.C.; Haarhaus, M. Association of Serum Sclerostin with Bone Sclerostin in Chronic Kidney Disease is Lost in Glucocorticoid Treated Patients. Calcif. Tissue Int. 2019, 104, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chekroun, A.; Pujo-Menjouet, L.; Falcoz, S.; Tsuen, K.; Yueh-Hsun Yang, K.; Berteau, J.P. Theoretical evidence of osteoblast self-inhibition after activation of the genetic regulatory network controlling mineralization. J. Theor. Biol. 2022, 537, 111005. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, s.; Supriyono, T.; Kurdi, O.; Tauviqirrahman, M.; Winarni, T.; Jamari, J. Tresca stress study of CoCrMo-on-CoCrMo bearings based on body mass index using 2D computational model. J. Tribol. 2022, 33, 31–38. [Google Scholar]
- Pant, A.; Paul, E.; Niebur, G.L.; Vahdati, A. Integration of mechanics and biology in computer simulation of bone remodeling. Prog. Biophys. Mol. Biol. 2021, 164, 33–45. [Google Scholar] [CrossRef]
- Boaretti, D.; Wehrle, E.; Bansod, Y.D.; Tourolle Né Betts, D.C.; Müller, R. Perspectives on in silico bone mechanobiology: Computational modelling of multicellular systems. Eur. Cells Mater. 2022, 44, 56–73. [Google Scholar] [CrossRef] [PubMed]

| Primer | Direction | Sequence |
|---|---|---|
| GAPDH GAPDH RANK RANK RANKL RANKL SOST SOST BMP2 BMP2 OPG OPG | Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse Forward Reverse | CCTGCCAAATATGATGACATCAAG GTGGTCGTTGAGGGCAATG ACGTGGACCCTTGCCCCAGT ACTGGCCACCAGGGGAGCTT CACCATCAGCTGAAGATAGT CCAAGATCTCTAACATGACG AGACCAAAGACGTGTCCGAG GGGATGCAGCGGAAGTC ATGGATTCGTGGTGGAAGTG GTGGAGTTCAGATGATCAGC GTAGGTGCCAGGAGCCATT CAATGAACAAGTGGCTGTGC |
| Control Group (n = 10) | Risedronate Group (n = 10) | p-Value | |
|---|---|---|---|
| Age (years) BMI (kg/m2) Hip BMD ASA score | 82.2 ± 4.1 23.19 ± 4.03 −3.19 ± 0.84 2.0 ± 0.82 | 81.1 ± 5.9 22.74 ± 2.58 −3.06 ± 0.82 1.9 ± 0.74 | 0.471 0.214 0.965 0.789 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-H.; Choi, E.-Y.; Jun, H.-S.; Kim, Y.-Y. Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study. Medicina 2022, 58, 1566. https://doi.org/10.3390/medicina58111566
Lee H-H, Choi E-Y, Jun H-S, Kim Y-Y. Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study. Medicina. 2022; 58(11):1566. https://doi.org/10.3390/medicina58111566
Chicago/Turabian StyleLee, Hwan-Hee, Eun-Yong Choi, Hyun-Sik Jun, and Young-Yul Kim. 2022. "Osteoclast and Sclerostin Expression in Osteocytes in the Femoral Head with Risedronate Therapy in Patients with Hip Fractures: A Retrospective Comparative Study" Medicina 58, no. 11: 1566. https://doi.org/10.3390/medicina58111566





