Emergence of High Antimicrobial Resistance among Critically Ill Patients with Hospital-Acquired Infections in a Tertiary Care Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Isolation and Identification of the Clinical Isolates
2.3. Antimicrobial Susceptibility Testing
2.4. Statistical Analysis
3. Results
3.1. Demographic Data of Included Patients
3.2. Prevalence of the Bacterial Isolates in Different Hospital Units
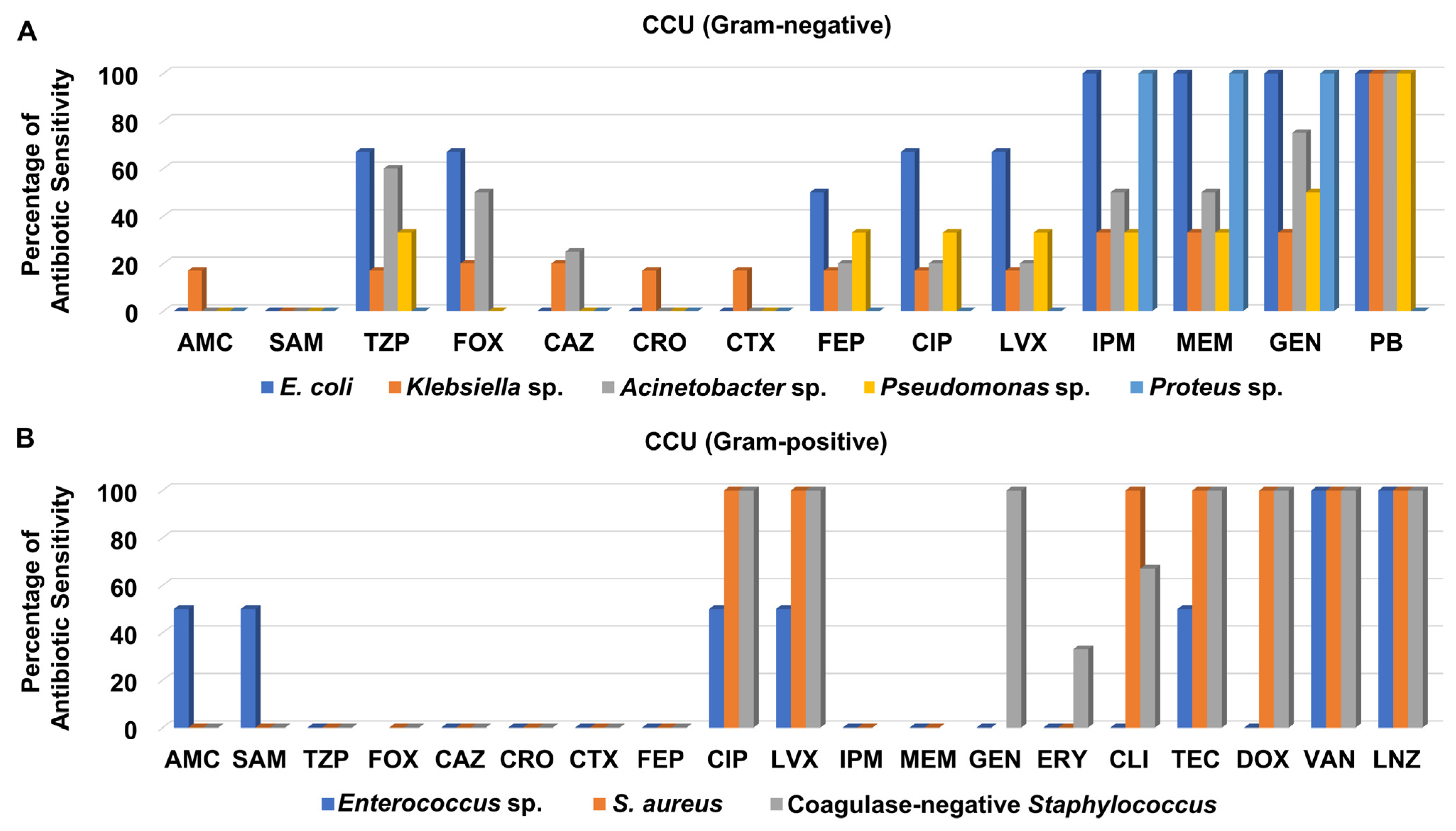
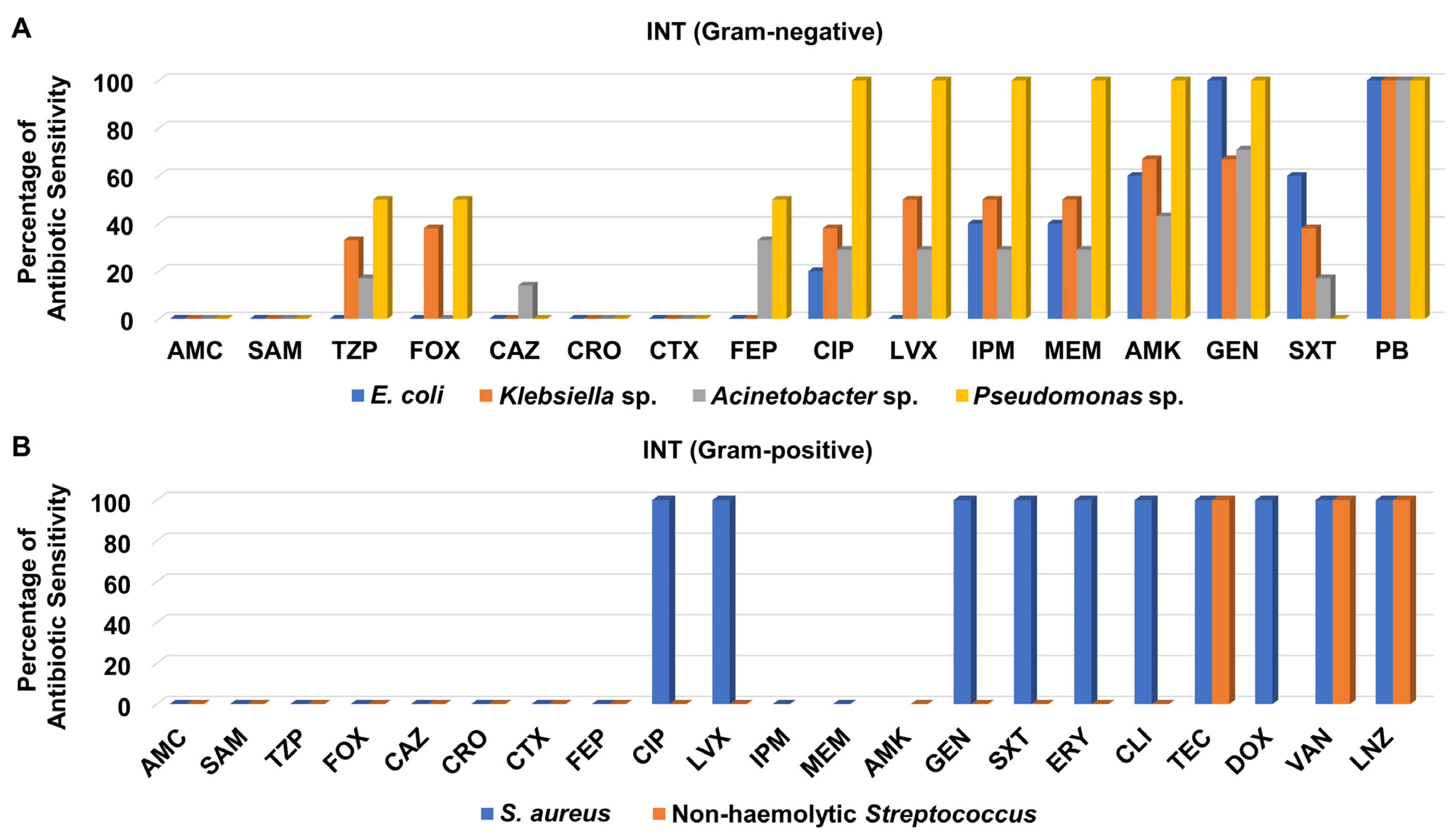
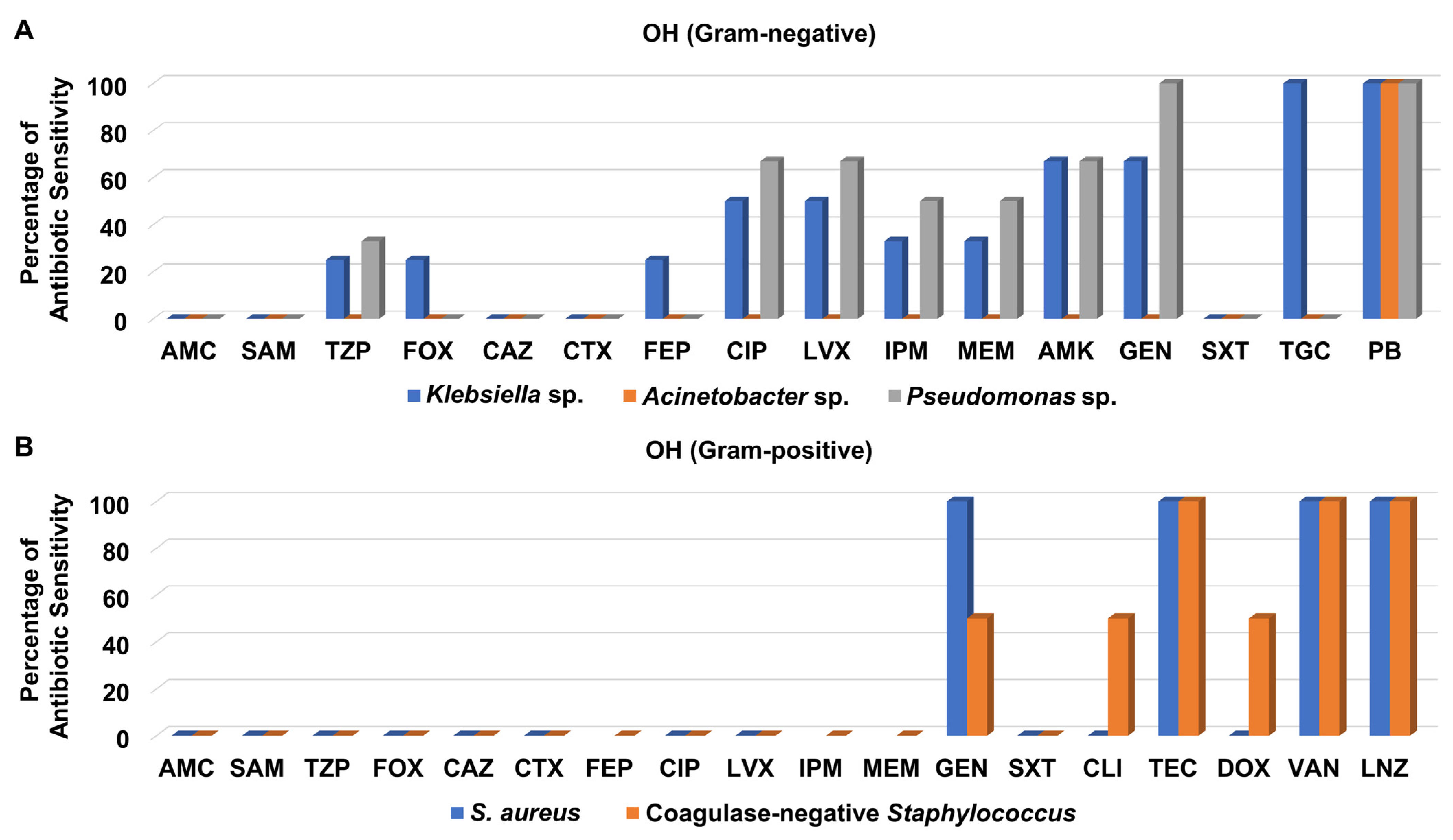
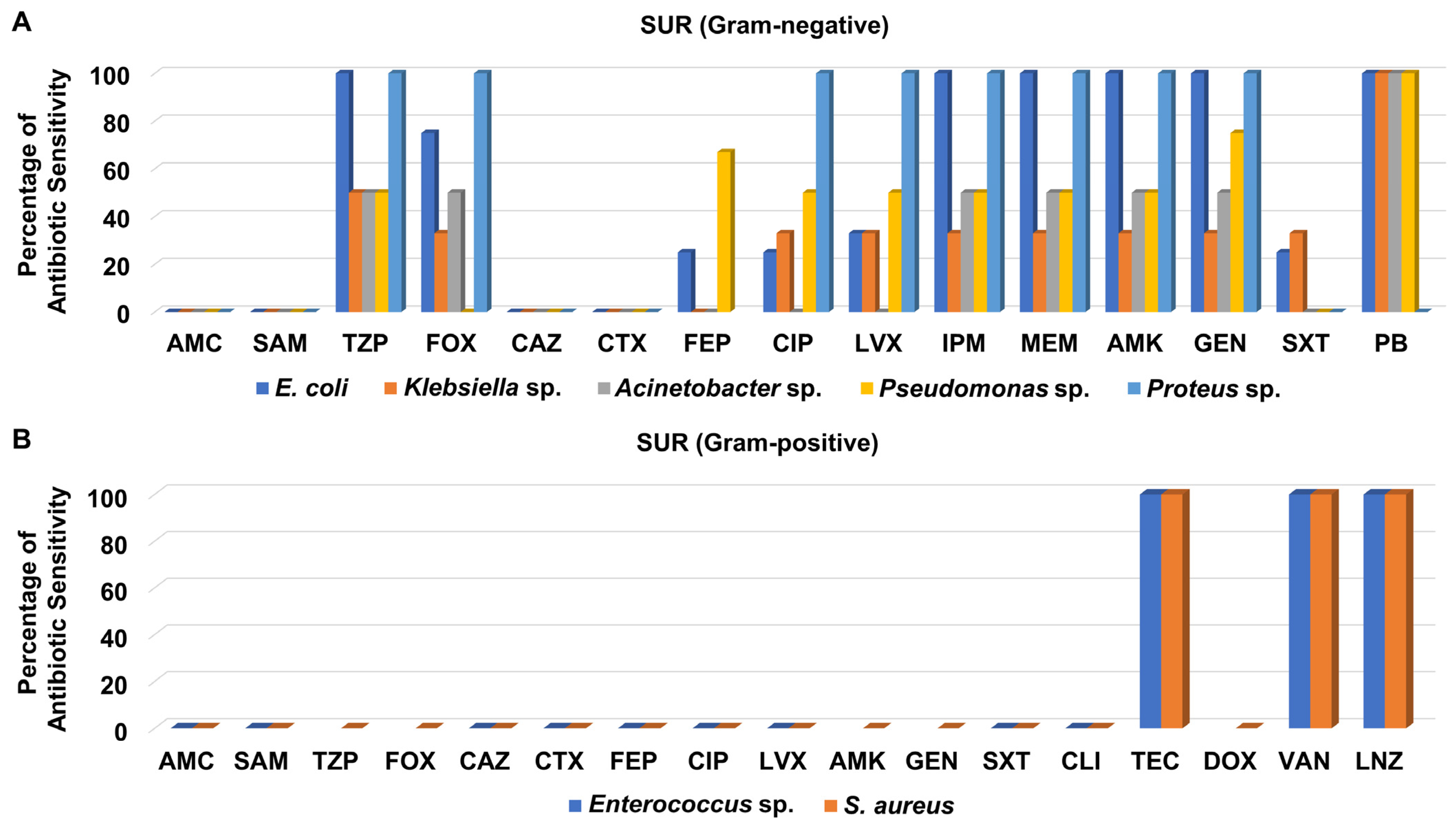
3.3. Antibiotic Sensitivity Pattern of Recovered Isolates from Different Hospital Units
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Health Care-Associated Infections Fact Sheet; World Health Organization: Geneva, Switzerland, 2015; p. 4. [Google Scholar]
- Rezai, M.-S.; Bagheri-Nesami, M.; Nikkhah, A. Catheter-related urinary nosocomial infections in intensive care units: An epidemiologic study in North of Iran. Casp. J. Intern. Med. 2017, 8, 76–82. [Google Scholar] [CrossRef]
- Mekonnen, H.; Seid, A.; Fenta, G.M.; Gebrecherkos, T. Antimicrobial resistance profiles and associated factors of Acinetobacter and Pseudomonas aeruginosa nosocomial infection among patients admitted at Dessie comprehensive specialized Hospital, North-East Ethiopia. A cross-sectional study. PLoS ONE 2021, 16, e0257272. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 2008, 36, 309–332. [Google Scholar] [CrossRef]
- Khawaja, A.; Arshad, F.; Munir, S. Acinetobacter species: Prevalence and sensitivity pattern of Acinetobacter species among clinical isolates of tertiary care hospital. Prof. Med. J. 2018, 25, 1949–1953. [Google Scholar]
- Warda, A.E.A.; Sarhan, R.M.; Al-Fishawy, H.S.; Moharram, A.N.; Salem, H.F. Continuous Versus Intermittent Linezolid Infusion for Critically Ill Patients with Hospital-Acquired and Ventilator-Associated Pneumonia: Efficacy and Safety Challenges. Pharmaceuticals 2022, 15, 296. [Google Scholar] [CrossRef]
- Brusselaers, N.; Vogelaers, D.; Blot, S. The rising problem of antimicrobial resistance in the intensive care unit. Ann. Intensiv. Care 2011, 1, 47. [Google Scholar] [CrossRef]
- Marwick, C.; Davey, P. Care bundles: The holy grail of infectious risk management in hospital? Curr. Opin. Infect. Dis. 2009, 22, 364–369. [Google Scholar] [CrossRef]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef]
- Carlet, J.; Ali, A.B.; Tabah, A.; Willems, V.; Philippar, F.; Chafine, A.; Misset, B. Multidrug resistant infections in the ICU: Mechanisms, prevention and treatment. In 25 Years of Progress and Innovation in Intensive Care Medicine; Kuhlen, R., Moreno, R., Ranieri, V.M., Rhodes , A., Eds.; Medizinisch Wissenschaftliche Verlagsgesellschaft: Berlin, Germany, 2007; pp. 199–211. [Google Scholar]
- Baym, M.; Stone, L.K.; Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science 2016, 351, aad3292. [Google Scholar] [CrossRef]
- Scaglione, V.; Reale, M.; Davoli, C.; Mazzitelli, M.; Serapide, F.; Lionello, R.; La Gamba, V.; Fusco, P.; Bruni, A.; Procopio, D.; et al. Prevalence of Antibiotic Resistance Over Time in a Third-Level University Hospital. Microb. Drug Resist. 2022, 28, 425–435. [Google Scholar] [CrossRef]
- Livermore, D.M. Antibiotic resistance during and beyond COVID-19. JAC-Antimicrob. Resist. 2021, 3, i5–i16. [Google Scholar] [CrossRef] [PubMed]
- Sulayyim, H.J.A.; Ismail, R.; Al Hamid, A.; Ghafar, N.A. Antibiotic Resistance during COVID-19: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 11931. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Flores, E.; Salcedo-Matienzo, J. Medicación prehospitalaria en pacientes hospitalizados por COVID-19 en un hospital público de Lima-Perú. Acta Médica Peru. 2020, 37, 393–395. [Google Scholar] [CrossRef]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020, 98, 442–442A. [Google Scholar] [CrossRef]
- Morales, E.; Cots, F.; Sala, M.; Comas, M.; Belvis, F.; Riu, M.; Salvadó, M.; Grau, S.; Horcajada, J.P.; Montero, M.M.; et al. Hospital costs of nosocomial multi-drug resistant Pseudomonas aeruginosa acquisition. BMC Health Serv. Res. 2012, 12, 122. [Google Scholar] [CrossRef]
- Huang, H.; Chen, B.; Liu, G.; Ran, J.; Lian, X.; Huang, X.; Wang, N.; Huang, Z. A multi-center study on the risk factors of infection caused by multi-drug resistant Acinetobacter baumannii. BMC Infect. Dis. 2018, 18, 11. [Google Scholar] [CrossRef]
- Hassan, M.A.; El-Aziz, S.A.; Elbadry, H.M.; El-Aassar, S.A.; Tamer, T.M. Prevalence, antimicrobial resistance profile, and characterization of multi-drug resistant bacteria from various infected wounds in North Egypt. Saudi J. Biol. Sci. 2022, 29, 2978–2988. [Google Scholar] [CrossRef]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Khoshbayan, A.; Chegini, Z.; Farahani, I.; Shariati, A. Bacteriophages, a New Therapeutic Solution for Inhibiting Multidrug-Resistant Bacteria Causing Wound Infection: Lesson from Animal Models and Clinical Trials. Drug Des. Dev. Ther. 2020, 14, 1867–1883. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- CLSI M100-S15; Performance Standards for Antimicrobial Susceptibility Testing, 28th Edition. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2018.
- Datta, P.; Gulati, N.; Singla, N.; Vasdeva, H.R.; Bala, K.; Chander, J.; Gupta, V. Evaluation of various methods for the detection of meticillin-resistant Staphylococcus aureus strains and susceptibility patterns. J. Med. Microbiol. 2011, 60, 1613–1616. [Google Scholar] [CrossRef] [PubMed][Green Version]
- WHONET Software. Available online: https://whonet.org/ (accessed on 16 March 2022).
- R-Project. Available online: https://www.r-project.org/about.html (accessed on 22 March 2022).
- Vincent, J.-L.; Rello, J.; Marshall, J.K.; Silva, E.; Anzueto, A.; Martin, C.D.; Moreno, R.; Lipman, J.; Gomersall, C.; Sakr, Y.; et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA 2009, 302, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Kaye, K.S.; Pogue, J.M. Infections Caused by Resistant Gram-Negative Bacteria: Epidemiology and Management. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobial Resistance Threats in the emerging COVID-19 pandemic: Where do we stand? J. Infect. Public Health 2021, 14, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Paquette, F.; Bernier-Jean, A.; Brunette, V.; Ammann, H.; Lavergne, V.; Pichette, V.; Troyanov, S.; Bouchard, J. Acute Kidney Injury and Renal Recovery with the Use of Aminoglycosides: A Large Retrospective Study. Nephron 2015, 131, 153–160. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Abramson, M.A.; Beekmann, S.E.; Gallagher, G.; Riedel, S.; Diekema, D.; Quinn, J.P.; Doern, G.V. Antimicrobial Resistance among Gram-Negative Bacilli Causing Infections in Intensive Care Unit Patients in the United States between 1993 and 2004. J. Clin. Microbiol. 2007, 45, 3352–3359. [Google Scholar] [CrossRef]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef]
- Gopalakrishnan, R.; Sureshkumar, D. Changing trends in antimicrobial susceptibility and hospital acquired infections over an 8 year period in a tertiary care hospital in relation to introduction of an infection control programme. J. Assoc. Physicians India 2010, 58, 25–31. [Google Scholar]
- Patel, B.V.; Patel, P.G.; Raval, P.N.; Patel, M.H.; Patel, P.H.; Vegad, M.M. Bacteriological profile and antibiogram of Gram negative organisms isolated from medical and neurology intensive care unit with special reference to multi-drug resistant organisms. Natl. J. Med. Res. 2012, 2, 335–338. [Google Scholar]
- Sharma, M.; Pathak, S.; Srivastava, P. Prevalence and antibiogram of Extended Spectrum β-Lactamase (ESBL) producing Gram negative bacilli and further molecular characterization of ESBL producing Escherichia coli and Klebsiella spp. J. Clin. Diagn. Res. 2013, 7, 2173. [Google Scholar] [CrossRef] [PubMed]
- Khullar, S.; Rathore, L.; Khatri, P.K.; Parihar, R.S.; Meena, S.; Bora, A.; Maurya, V.; Sharma, N. Identification and Antibiogram of Various Gram Positive Bacterial Isolates from Pyogenic Samples by VITEK® 2 Compact System. Int. J. Curr. Microbiol. Appl. Sci. 2016, 5, 66–77. [Google Scholar] [CrossRef]
- Soltani, R.; Khalili, H.; Abdollahi, A.; Rasoolinejad, M.; Dashti-Khavidaki, S. Nosocomial Gram-positive antimicrobial susceptibility pattern at a referral teaching hospital in Tehran, Iran. Futur. Microbiol. 2012, 7, 903–910. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alzahrani, A.J.; Tobaiqy, M.; Alresasi, A.M.; Bu-Shehab, I.; Al-Hadary, I.; Alhmeed, N.; Alismail, M.; et al. Antimicrobial susceptibility of gram-positive and gram-negative bacteria: A 5-year retrospective analysis at a multi-hospital healthcare system in Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mir, B.A.; Srikanth. Prevalence and Antimicrobial Susceptibility of methicillin resistant Staphylococcus aureus and coagulase-negative Staphylococci in a tertiary care hospital. Asian J. Pharm. Clin. Res. 2013, 6, 231–234. [Google Scholar]
- Pai, V.; I Rao, V.; Rao, S.P. Prevalence and Antimicrobial Susceptibility Pattern of Methicillin-resistant Staphylococcus Aureus [MRSA] Isolates at a Tertiary Care Hospital in Mangalore, South India. J. Lab. Physicians 2010, 2, 082–084. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Poirel, L.; A Bonomo, R.; Schwaber, M.J.; Daikos, G.L.; Cormican, M.; Cornaglia, G.; Garau, J.; Gniadkowski, M.; Hayden, M.K.; et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect. Dis. 2013, 13, 785–796. [Google Scholar] [CrossRef]
- Neuhauser, M.M.; Weinstei, R.A.; Rydman, R.; Danziger, L.H.; Karam, G.; Quinn, J.P. Antibiotic resistance among Gram-negative bacilli in US intensive care units: Implications for fluoroquinolone use. JAMA 2003, 289, 885–888. [Google Scholar] [CrossRef]
- Elkolaly, R.M.; Bahr, H.M.; El-Shafey, B.I.; Basuoni, A.S.; Elber, E.H. Incidence of ventilator-associated pneumonia: Egyptian study. Egypt. J. Bronchol. 2019, 13, 258–266. [Google Scholar] [CrossRef]
- Negm, E.M.; Mowafy, S.M.S.; Mohammed, A.A.; Amer, M.G.; Tawfik, A.E.; Ibrahim, A.E.S.; Hassan, T.H. Antibiograms of intensive care units at an Egyptian tertiary care hospital. Egypt. J. Bronchol. 2021, 15, 1–15. [Google Scholar] [CrossRef]
- Qadeer, A.; Akhtar, A.; Ain, Q.U.; Saadat, S.; Mansoor, S.; Assad, S.; Ishtiaq, W.; Ilyas, A.; Khan, A.Y.; Ajam, Y. Antibiogram of Medical Intensive Care Unit at Tertiary Care Hospital Setting of Pakistan. Cureus 2016, 8, e809. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Rao, A. Antibiogram of Gram-negative bacterial isolates from intensive care unit at a tertiary care hospital. IJAR 2016, 6, 344–347. [Google Scholar]
- Sheth, K.; Patel, T.; Malek, S.; Tripathi, C. Antibiotic Sensitivity Pattern of Bacterial Isolates from the Intensive Care Unit of a Tertiary Care Hospital in India. Trop. J. Pharm. Res. 2013, 11, 991–999. [Google Scholar] [CrossRef]
- Gunjal, P.N.; Gunjal, S.; Kher, S. A Cross-sectional study to determine the profile and antibiotic resistance pattern of gram negative bacilli isolated from intensive care unit patients in a tertiary care hospital in ahmednagar, maharashtra. Int. J. Biomed. Adv. Res. 2012, 3, 281–284. [Google Scholar] [CrossRef]
- Khan, M.A. Bacterial spectrum and susceptibility patterns of pathogens in ICU and IMCU of a secondary care hospital in Kingdom of Saudi Arabia. Int. J. Pathol. 2018, 16, 64–70. [Google Scholar]
- Raakhee, T.; Rao, U.S. Prevalence and resistance pattern of Pseudomonas strains isolated from ICU patients. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 527–534. [Google Scholar]
- Radji, M.; Fauziah, S.; Aribinuko, N. Antibiotic sensitivity pattern of bacterial pathogens in the intensive care unit of Fatmawati Hospital, Indonesia. Asian Pac. J. Trop. Biomed. 2011, 1, 39–42. [Google Scholar] [CrossRef]
- Savanur, S.S.; Gururaj, H. Study of Antibiotic Sensitivity and Resistance Pattern of Bacterial Isolates in Intensive Care Unit Setup of a Tertiary Care Hospital. Indian J. Crit. Care Med. 2019, 23, 547–555. [Google Scholar] [CrossRef]
- Vanitha, R.N.; Gopal, K.; Narendra, M.V.; Vishwakanth, D. A retrospective study on blood stream infections and antibiotic susceptibility patterns in a tertiary care teaching hospital. Int. J. Pharm. Pharm. Sci. 2012, 4, 543–548. [Google Scholar]
- Chidambaram, N.; Ambujam, G.; Rajan, R.; Sasikala, G.; Anandi, V. Antimicrobial profile of clinical isolates in intensive care unit at a tertiary care hospital. Int. J. Med. Res. Health Sci. 2019, 8, 160–166. [Google Scholar]
- Kaur, A.; Singh, V.A. Bacterial isolates and their antibiotic sensitivity pattern in clinically suspected cases of fever of unknown origin. JK Sci. 2014, 16, 105. [Google Scholar]
- Davoudi, A.; Najafi, N.; Alian, S.; Tayebi, A.; Ahangarkani, F.; Rouhi, S.; Heydari, A. Resistance Pattern of Antibiotics in Patient Underwent Open Heart Surgery With Nosocomial Infection in North of Iran. Glob. J. Health Sci. 2015, 8, 288–297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parameswarappa, J.; Metri, B.V.P.B.C. Isolation, identification, and antibiogram of Enterococci isolated from patients with urinary tract infection. Ann. Afr. Med. 2013, 12, 176–181. [Google Scholar] [PubMed]
- Almqvist, M.; Mattsson, G.; Razmi, R.; Magnusson, P. Cardiac Implantable Electronic Device-Related Infections; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Seni, J.; Najjuka, C.F.; Kateete, D.P.; Kateete, P.; Joloba, M.L.; Kajumbula, H.; Kapesa, A.; Bwanga, F. Antimicrobial resistance in hospitalized surgical patients: A silently emerging public health concern in Uganda. BMC Res. Notes 2013, 6, 1–7. [Google Scholar] [CrossRef]
- Agaba, P.; Tumukunde, J.; Tindimwebwa, J.V.B.; Kwizera, A. Nosocomial bacterial infections and their antimicrobial susceptibility patterns among patients in Ugandan intensive care units: A cross sectional study. BMC Res. Notes 2017, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Valsamatzi-Panagiotou, A.; Popova, K.B.; Penchovsky, R. Strategies for Prevention and Containment of Antimicrobial Resistance; Sustainable Agriculture Reviews 49; Springer: Cham, Switzerland, 2021; pp. 1–31. [Google Scholar] [CrossRef]
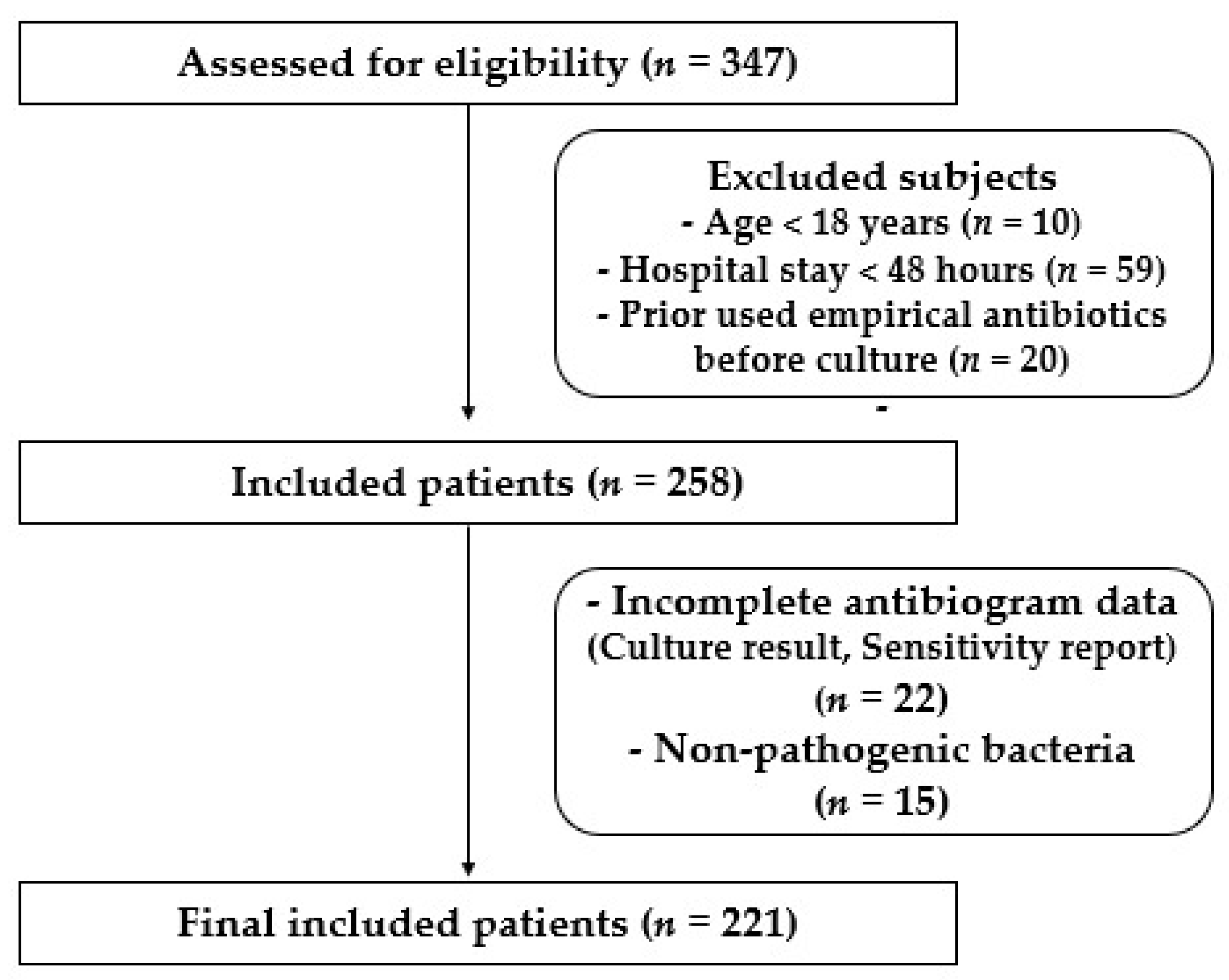
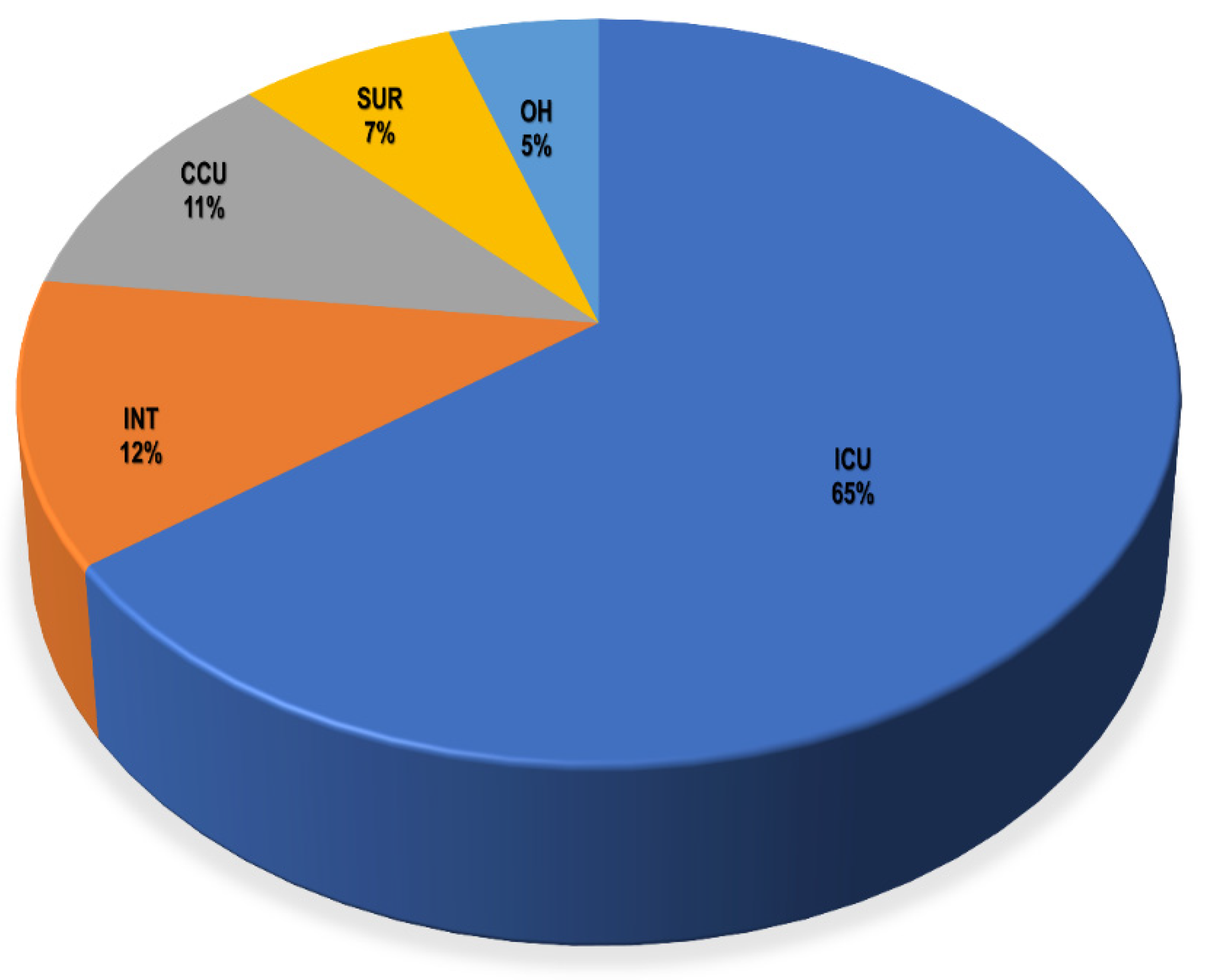
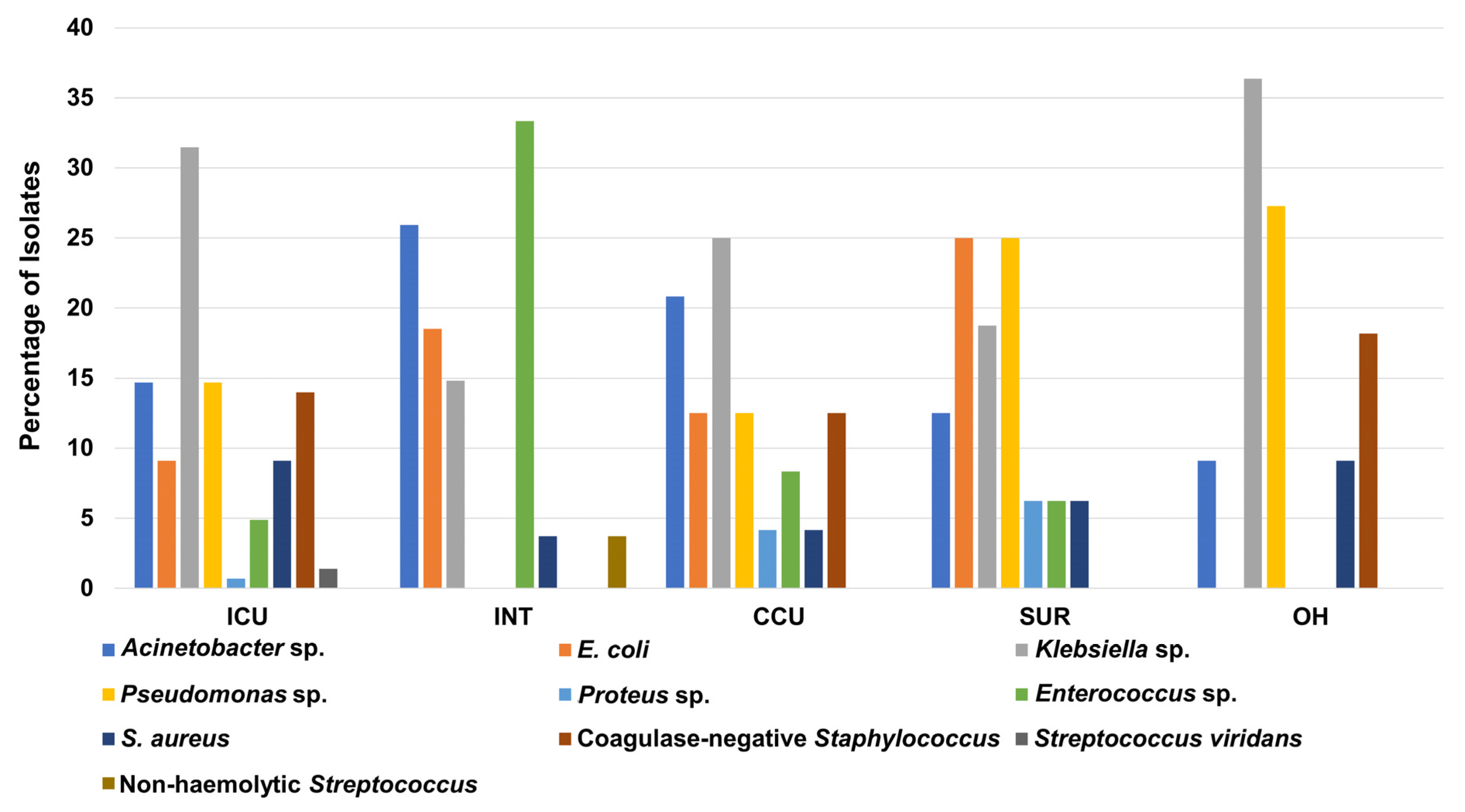
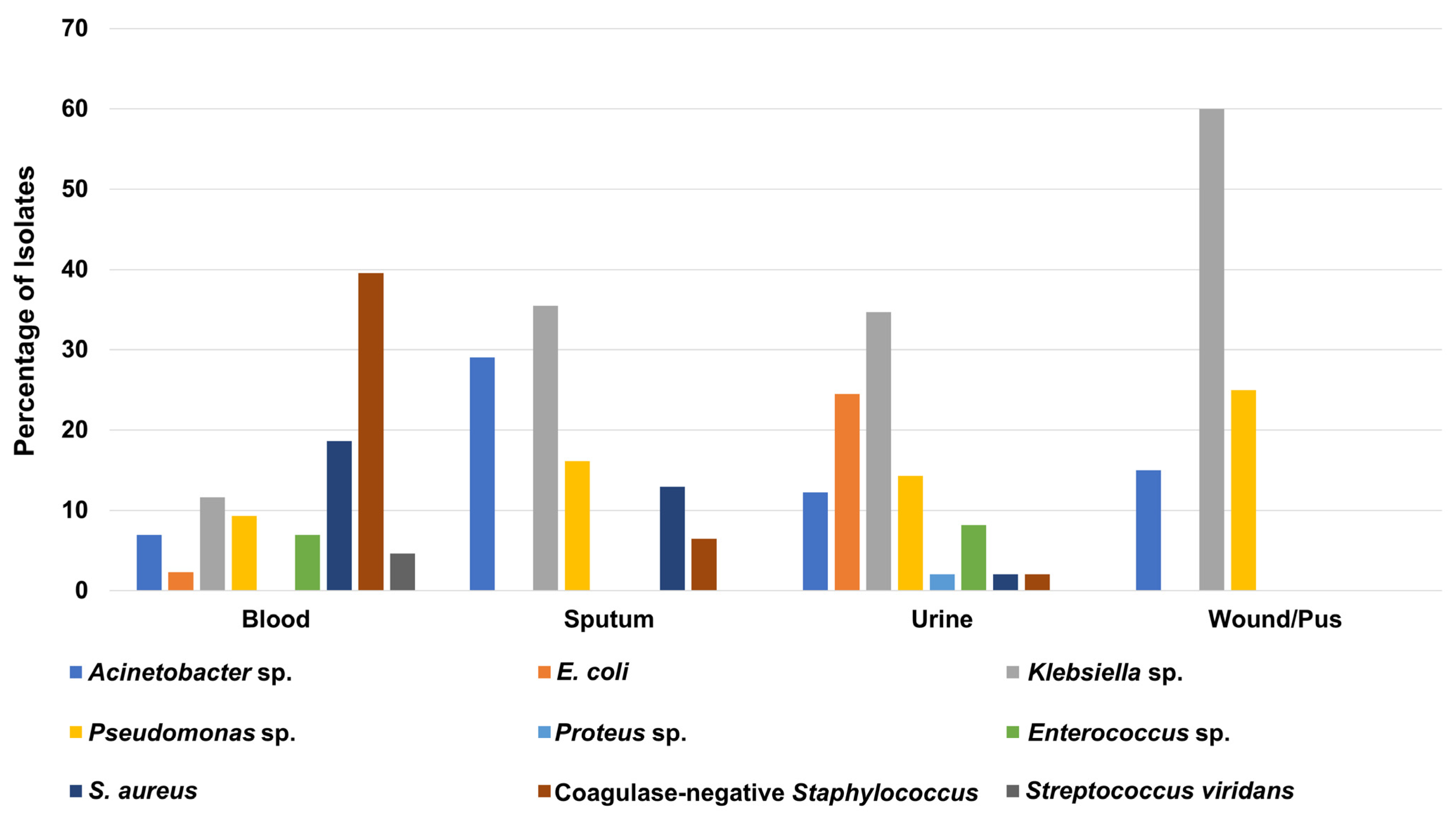
| Parameters | Total (n = 221) | ICU (n = 143) | CCU (n = 24) | OH (n = 11) | INT (n = 27) | SUR (n = 16) |
|---|---|---|---|---|---|---|
| Gender, n% | ||||||
| Male | 156 (70.6%) | 113 (79.0%) | 17 (70.8%) | 6 (54.4%) | 19 (70.4%) | 14 (87.5%) |
| Female | 65 (29.4%) | 30 (21.0%) | 7 (29.2%) | 5 (45.5%) | 8 (29.6%) | 2 (12.5%) |
| Age (years), n% | ||||||
| 18–39 | 34 (15.4%) | 34 (23.8%) | 4 (16.7%) | 0 (0%) | 6 (22.2%) | 2 (12.5%) |
| 40–60 | 60 (57.1%) | 60 (42.0%) | 8 (33.3%) | 2 (18.2%) | 5 (18.5%) | 8 (50.0%) |
| >60 | 127 (57.5%) | 49 (34.3%) | 12 (50.0%) | 9 (81.8%) | 16 (59.3%) | 6 (37.5%) |
| Median (IQR) | 65 (49–76.5) | 55 (40–64) | 47.5 (47–72.5) | 74 (68–78) | 63 (50–79) | 55 (44.25–70.5) |
| Type of infection, n% | ||||||
| HAP | 32 (14.5%) | 18 (12.6%) | 5 (20.8%) | 1 (9.1%) | 3 (11.1%) | 5 (31.3%) |
| VAP | 24 (10.9%) | 13 (9.1%) | 5 (20.8%) | 3 (27.3%) | 1 (3.7%) | 2 (12.5%) |
| UTI | 75 (33.9%) | 50 (35.0%) | 6 (25.0%) | 2 (18.2%) | 13 (48.1%) | 4 (25.0%) |
| BSI | 32 (14.5%) | 23 (16.1%) | 2 (8.3%) | 2 (18.2%) | 3 (11.1%) | 2 (12.5%) |
| CRBSI | 25 (11.3%) | 21 (14.7%) | 1 (4.2%) | 1 (9.1%) | 2 (7.4%) | 0 (0%) |
| SSTI | 23 (10.4%) | 10 (7.0%) | 5 (20.8%) | 2 (18.2%) | 3 (11.1%) | 3 (18.8%) |
| IAI | 10 (4.5%) | 8 (5.6%) | 0 (0%) | 0 (0%) | 2 (7.4%) | 0 (0%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
E. Abou Warda, A.; Molham, F.; Salem, H.F.; Mostafa-Hedeab, G.; ALruwaili, B.F.; Moharram, A.N.; Sebak, M.; Sarhan, R.M. Emergence of High Antimicrobial Resistance among Critically Ill Patients with Hospital-Acquired Infections in a Tertiary Care Hospital. Medicina 2022, 58, 1597. https://doi.org/10.3390/medicina58111597
E. Abou Warda A, Molham F, Salem HF, Mostafa-Hedeab G, ALruwaili BF, Moharram AN, Sebak M, Sarhan RM. Emergence of High Antimicrobial Resistance among Critically Ill Patients with Hospital-Acquired Infections in a Tertiary Care Hospital. Medicina. 2022; 58(11):1597. https://doi.org/10.3390/medicina58111597
Chicago/Turabian StyleE. Abou Warda, Ahmed, Fatma Molham, Heba F. Salem, Gomaa Mostafa-Hedeab, Bashayer F. ALruwaili, Ayman N. Moharram, Mohamed Sebak, and Rania M. Sarhan. 2022. "Emergence of High Antimicrobial Resistance among Critically Ill Patients with Hospital-Acquired Infections in a Tertiary Care Hospital" Medicina 58, no. 11: 1597. https://doi.org/10.3390/medicina58111597
APA StyleE. Abou Warda, A., Molham, F., Salem, H. F., Mostafa-Hedeab, G., ALruwaili, B. F., Moharram, A. N., Sebak, M., & Sarhan, R. M. (2022). Emergence of High Antimicrobial Resistance among Critically Ill Patients with Hospital-Acquired Infections in a Tertiary Care Hospital. Medicina, 58(11), 1597. https://doi.org/10.3390/medicina58111597







