Robotic-Arm-Assisted Total Hip Arthroplasty: A Review of the Workflow, Outcomes and Its Role in Addressing the Challenge of Spinopelvic Imbalance
Abstract
1. Introduction
2. The Spinopelvic Challenge
3. Workflow
3.1. Pre-Operative Planning and Preparation
3.2. Intra-Operative Workflow
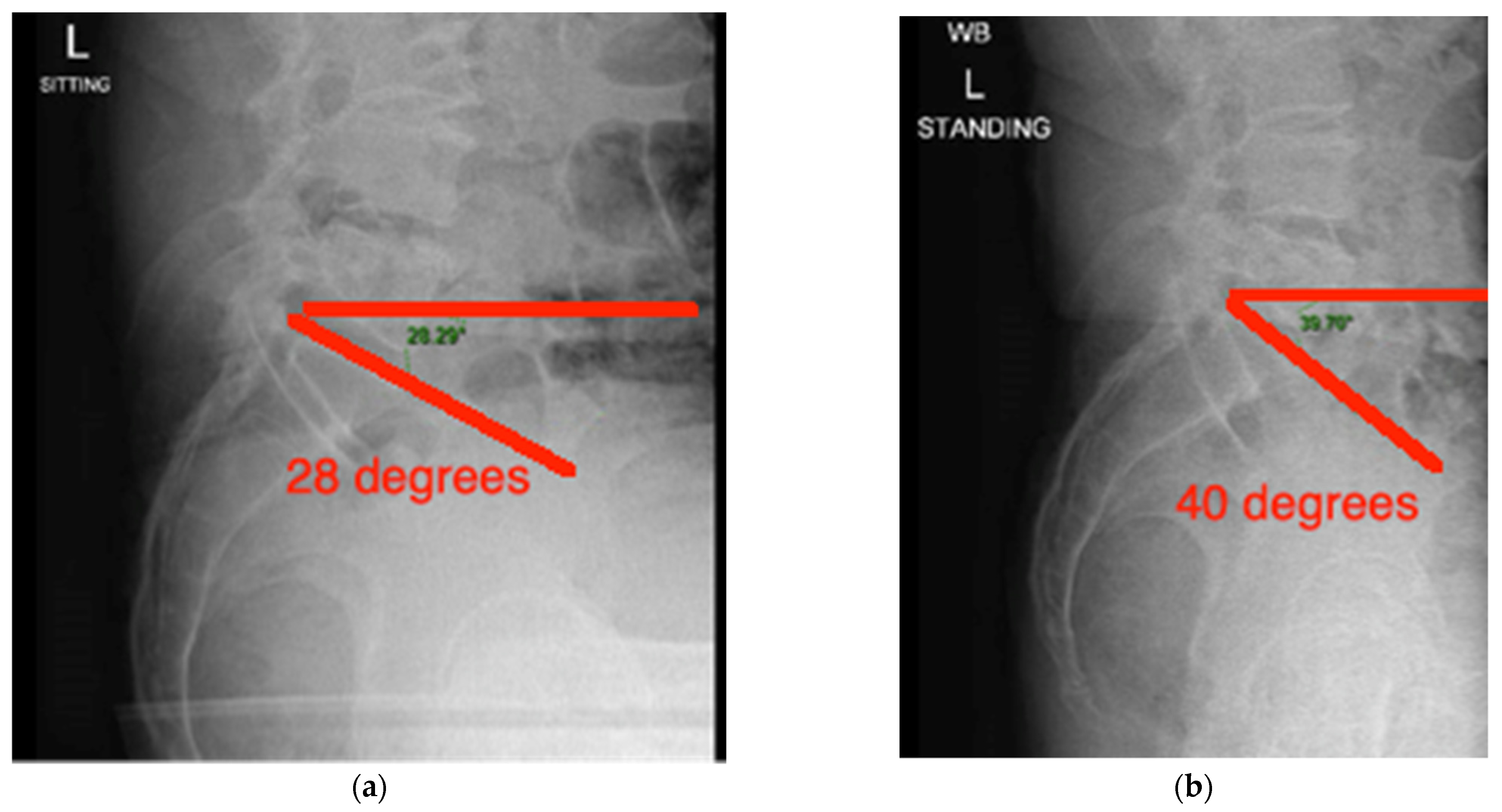
3.3. Case Presentation
- The patient had previously undergone a left total hip replacement complicated by two post-operative dislocations. She did not report a history of back pain.
- The difference in sacral slope between standing and sitting radiographs was noted to be 6 degrees. According to the Stefl classification, this is stuck sitting, as the sacral tilt does not tilt anteriorly beyond 30 degrees with standing, indicating a high-risk patient (Figure 6a–c).
- In this case, the native femoral retroversion (−6 degrees) posed a challenge in avoiding impingement (Figure 7). Upon assessing VROM, bone-on-bone and implant-on-implant impingement in deep flexion were noted (Figure 8). Using the robotic software, the planned femoral version was corrected to +16 in the femoral broach (Figure 9a,b).
- VROM was performed again, and impingement in flexion was eliminated (Figure 10). In extension, upon subtracting the femur, it became apparent that there was a small area of impingement secondary to an anterior osteophyte which was planned to be removed after cup insertion during the operation (Figure 11a,b).
- The robotic software also enables preoperative and intraoperative visualisation of the anticipated postoperative X-rays accounting for any changes to the plan. In addition, the software allows for calculation of changes to the leg length offset compared to the preoperative and contralateral hips. In this case, the leg length was 1 mm longer compared to the opposite hip, and the combined offset was 6 mm increased compared to that preoperatively (Figure 12a,b).
4. Outcomes
4.1. Component Accuracy
4.2. PROMs
4.3. Complications
4.4. Cost Efficacy
| Author and Year | Material and Methods | Research Type | Measured Outcomes | Key Results |
|---|---|---|---|---|
| Han et al. [28] 2019 | From Embase, PubMed, Cochrane Library 14 studies included: 12 high quality and 2 medium quality | Systematic review and meta-analysis | Comparing functional outcomes, radiological outcomes and complication rates between CoTHA and RoTHA | CoTHA had less case of dislocations compared to RoTHA. WOMAC, HHS and Merle D’Aubigne Hip Score showed no statistical difference. Robotic THA resulted in greater number of implants in Lewinnek’s safe zone |
| Domb et al. [27] 2020 | From total of 217 patients, 66 patients matched into each cohort (RoTHA and CoTHA). | Propensity Score-Matched study | Comparing PROMs, acetabular implant placement, survivorship and complications between each cohort. | RoTHA resulted in improved PROMs (HHS FJS-12, VR-12 Physical, SR-12) compared to CoTHA. Improved implant accuracy within Lewinnek with RoTHA vs. CoTHA (97% and 73.8% respectively) |
| Clement et al. [26] 2020 | 40 RoTHA patients and 80 CoTHA patients, performed by single surgeon | Propensity Score-Matched study | Comparing PROMs, implant positioning, patient satisfaction and restoration of leg length | Statistically significant improvement in OHS score of 2.5 95% CI 0.1–4.8 p = 0.038 comparing RoTHA to CoTHA. 97.5% of RoTHA components within Lewinnek and Callanan’s safe zone |
| Ng et al. [5] 2021 | Search yielded 510 articles from Medline, PubMed and Google Scholar. 17 included | Systematic review and meta-analysis | Report on learning curve, compare implant positioning, survivorship of implants, functional outcomes and complications between semi-active RoTHA and CoTHA | Implant accuracy in RoTHA between 77% and 100%, whereas CoTHA between 30% and 82%. Statistically significant improvement in HHS with a mean difference of 3.05 95% CI 0.46 to 5.64 |
| Samuel et al. [30] 2021 | Search yielded 526 studies from PubMed, Embase and Cochrane Library. 18 included | Systematic review and meta-analysis | Comparing PROMs, dislocation, infection and revision rates between RoTHA (MAKO and ROBODOC) and CoTHA. | No significant difference in HHS, FJS, SF scores, Merle d’Aubigne but statistically significant improvement in WOMAC MD: −3.57 95% CI −5.62 to −1.52 p = 0.006. No difference in revision rates No difference in dislocation rates, but statistical difference when comparing ROBODOC to CoTHA. |
| Chen et al. [32] 2018 | Search yielded 178 studies from Medline, Embase, Cochrane Library and other manual sources. 8 included. | Systematic review and meta-analysis | Comparing surgical times, PROMs, complications and radiographic outcomes between RoTHA and CoTHA | Intraoperative complications significantly higher in CoTHA than RoTHA with similar post-operative complication rates. No significant difference in PROMs score, surgical times or limb length discrepancies. Improved component accuracy. |
| Karunaratne et al. [29] 2019 | Search yielded 2957 articles from PubMed, Medline, Embase and CENTRAL | Systematic review and meta-analysis | Comparing RoTHA and RoTKA PROMs of both fully active and semi-active against conventional. | No significant difference in PROMs for both fully active and semi-active RoTHA compared to CoTHA. |
| Kayani et al. [11] 2019 | 50 CoTHA and 25 RoTHA patients, single surgeon | Cohort study | Comparing accuracy in restoring native centre of rotation, planned combined offset, component accuracy and leg length correction between RoTHA and CoTHA. | RoTHA associated with improved accuracy in restoring native hip centres of rotation, improved preservation of native combined offset and acetabular component accuracy. |
| Maldonado et al. [36] 2021 | 555 patients who underwent RoTHA Utilised Markov model | Cost effectiveness study utilising a Markov model | To assess the QALY and cost of RoTHA vs. CoTHA | RoTHA produces more QALY compared to CoTHA (2.96 ± 0.58 and 2.92 ± 0.57) RoTHA Medicare patients was $945 less than CoTHA patients and $1810 less for private insurance patients at 5 years. |
| Kirchner et al. [37] 2021 | 758 RoTHA matched against 758 CoTHA | Retrospective cohort analysis | To assess cost of inpatient care | Average inpatient cost for RoTHA $20,046 ± 6165 compared to CoTHA $18,258 ± 6147. |
| Bendich et al. [34] 2022 | 13,802 posterior approach THAs (1770 RoTHA, 3155 computer navigated THA, 8877 CoTHA) | Retrospective cohort analysis | To assess rates of complication | Lower risk of revision surgery for dislocation at 1 year post primary index surgery with RoTHA compared to CoTHA with Odds Ratio of 0.3. |
| Shaw et al. [35] 2022 | 2247 patients (1724 CoTHA and 523 RoTHA), 3 surgeons | Retrospective cohort analysis | To compare pre-operative, post operative PROMs and complication rates between CoTHA and RoTHA | No difference in PROMs (PROMIS-GH, PROMIS-MH, PROMIS-PH and HOOS, JR), reduced risk of dislocation |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mart, J.P.S.; Goh, E.L.; Shah, Z. Robotics in total hip arthroplasty: A review of the evolution, application and evidence base. EFORT Open Rev. 2020, 5, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Fontalis, A.; Kayani, B.; Thompson, J.W.; Plastow, R.; Haddad, F.S. Robotic total hip arthroplasty: Past, present and future. Orthop. Trauma 2022, 36, 6–13. [Google Scholar] [CrossRef]
- Kiss, L. 75th anniversary of the death of Karel Čapek, the Czech writer and creator of the term “robot”. Orv. Hetil. 2013, 154, 2005–2007. [Google Scholar] [CrossRef]
- Honl, M.; Dierk, O.; Gauck, C.; Carrero, V.; Lampe, F.; Dries, S.; Quante, M.; Schwieger, K.; Hille, E.; Morlock, M.M. Comparison of robotic-assisted and manual implantation of a primary total hip replacement. J. Bone Jt. Surg. 2003, 85, 1470–1478. [Google Scholar] [CrossRef]
- Ng, N.; Gaston, P.; Simpson, P.M.; Macpherson, G.J.; Patton, J.T.; Clement, N.D. Robotic arm-assisted versus manual total hip arthroplasty. Bone Jt. J. 2021, 103, 1009–1020. [Google Scholar] [CrossRef]
- Sultan, A.A.; Khlopas, A.; Piuzzi, N.S.; Chughtai, M.; Sodhi, N.; Mont, M.A. The Impact of Spino-Pelvic Alignment on Total Hip Arthroplasty Outcomes: A Critical Analysis of Current Evidence. J. Arthroplast. 2018, 33, 1606–1616. [Google Scholar] [CrossRef]
- Eftekhary, N.; Shimmin, A.; Lazennec, J.Y.; Buckland, A.; Schwarzkopf, R.; Dorr, L.D.; Mayman, D.; Padgett, D.; Vigdorchik, J. A systematic approach to the hip-spine relationship and its applications to total hip arthroplasty. Bone Jt. J. 2019, 101-B, 808–816. [Google Scholar] [CrossRef]
- Hayashi, S.; Hashimoto, S.; Kuroda, Y.; Nakano, N.; Matsumoto, T.; Ishida, K.; Shibanuma, N.; Kuroda, R. Robotic-arm assisted THA can achieve precise cup positioning in developmental dysplasia of the hip. Bone Jt. Res. 2021, 10, 629–638. [Google Scholar] [CrossRef]
- Abdel, M.P.; Von Roth, P.; Jennings Bs, M.T.; Hanssen, A.D.; Pagnano, M.W. What Safe Zone? The Vast Majority of Dislocated THAs Are Within the Lewinnek Safe Zone for Acetabular Component Position. Clin. Orthop. Relat. Res. 2006, 474, 386–391. [Google Scholar] [CrossRef]
- Seagrave, K.G.; Troelsen, A.; Malchau, H.; Husted, H.; Gromov, K. Acetabular cup position and risk of dislocation in primary total hip arthroplasty: A systematic review of the literature. Acta Orthop. 2017, 88, 10. [Google Scholar] [CrossRef]
- Kayani, B.; Konan, S.; Ayuob, A.; Ayyad, S.; Haddad, F.S. The current role of robotics in total hip arthroplasty. EFORT Open Rev. 2019, 4, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, N.; Trasolini, N.A.; Stefl, M.; Dorr, L. The Effect of Spinopelvic Motion on Implant Positioning and Hip Stability Using the Functional Safe Zone of THR. In Personalized Hip and Knee Joint Replacement; Springer: Berlin/Heidelberg, Germany, 2020; pp. 133–142. [Google Scholar]
- Larkin, B.; van Holsbeeck, M.; Koueiter, D.; Zaltz, I. What is the impingement-free range of motion of the asymptomatic hip in young adult males? Clin. Orthop. Relat. Res. 2015, 473, 1284–1288. [Google Scholar] [CrossRef]
- Gorin, M.; Roger, B.; Lazennec, J.-Y.; Charlot, N.; Arafati, N.; Bissery, A.; Saillant, G. Hip-spine relationship: A radio-anatomical study for optimization in acetabular cup positioning. Surg. Radiol. Anat. 2003, 26, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Klemt, C.; Limmahakhun, S.; Bounajem, G.; Xiong, L.; Yeo, I.; Kwon, Y.M. Effect of postural changes on in vivo pelvic tilt and functional component anteversion in total hip arthroplasty patients with lumbar disc degenerations. Bone Jt. J. 2020, 102, 1505–1510. [Google Scholar] [CrossRef]
- Bracey, D.N.; Hegde, V.; Shimmin, A.J.; Jennings, J.M.; Pierrepont, J.W.; Dennis, D.A. Spinopelvic mobility affects accuracy of acetabular anteversion measurements on cross-table lateral radiographs. Bone Jt. J. 2021, 103-B, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Stefl, M.; Lundergan, W.; Heckmann, N.; McKnight, B.; Ike, H.; Murgai, R.; Dorr, L.D. Spinopelvic mobility and acetabular component position for total hip arthroplasty. Bone Jt. J. 2017, 99 (Suppl. A), 37–45. [Google Scholar] [CrossRef] [PubMed]
- Vigdorchik, J.M.; Sharma, A.K.; Buckland, A.J.; Elbuluk, A.M.; Eftekhary, N.; Mayman, D.J.; Carroll, K.M.; Jerabek, S.A. 2021 Otto Aufranc Award: A simple Hip-Spine Classification for total hip arthroplasty. Bone Jt. J. 2021, 103-B, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Florio, J. Why Do We Say Measure Twice Cut Once? Second Frutes. 1591. Available online: https://www.bookbrowse.com/expressions/detail/index.cfm/expression_number/14/measure-twice-cut-once (accessed on 9 August 2022).
- Lewinnek, G.E.; Lewis, J.L.; Tarr, R.; Compere, C.L.; Zimmerman, J.R. Dislocations after total hip-replacement arthroplasties. J. Bone Jt. Surg. 1978, 60, 217–220. [Google Scholar] [CrossRef]
- Callanan, M.C.; Jarrett, B.; Bragdon, C.R.; Zurakowski, D.; Rubash, H.E.; Freiberg, A.A.; Malchau, H. The John Charnley Award: Risk factors for cup malpositioning: Quality improvement through a joint registry at a tertiary hospital. Clin. Orthop. Relat. Res. 2011, 469, 319–329. [Google Scholar] [CrossRef]
- Ranawat, C.S.; Maynard, M.J. Modern technique of cemented total hip arthroplasty. Tech. Orthop. 1991, 6, 17–25. [Google Scholar] [CrossRef]
- Dorr, L.D.; Malik, A.; Dastane, M.; Wan, Z. Combined Anteversion Technique for Total Hip Arthroplasty. Clin. Orthop. Relat. Res. 2009, 467, 119. [Google Scholar] [CrossRef] [PubMed]
- Rowan, F.E.; Benjamin, B.; Pietrak, J.R.; Haddad, F.S. Prevention of Dislocation After Total Hip Arthroplasty. J. Arthroplast. 2018, 33, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Kayani, B.; Konan, S.; Thakrar, R.R.; Huq, S.S.; Haddad, F.S. Assuring the long-term total joint arthroplasty: A triad of variables. Bone Jt. J. 2019, 101, 11–18. [Google Scholar] [CrossRef]
- Clement, N.D.; Gaston, P.; Bell, A.; Simpson, P.; Macpherson, G.; Hamilton, D.F.; Patton, J.T. Robotic arm-assisted versus manual total hip arthroplasty: A propensity score matched cohort study. Bone Jt. Res. 2021, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Domb, B.G.; Chen, J.W.; Lall, A.C.; Perets, I.; Maldonado, D.R. Minimum 5-Year Outcomes of Robotic-assisted Primary Total Hip Arthroplasty With a Nested Comparison Against Manual Primary Total Hip Arthroplasty: A Propensity Score-Matched Study. J. Am. Acad. Orthop. Surg. 2020, 28, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Han, P.F.; Chen, C.L.; Zhang, Z.L.; Han, Y.C.; Wei, L.; Li, P.C.; Wei, X.C. Robotics-assisted versus conventional manual approaches for total hip arthroplasty: A systematic review and meta-analysis of comparative studies. Int. J. Med. Robot. Comput. Assist. Surg. 2019, 15, e1990. [Google Scholar] [CrossRef]
- Karunaratne, S.; Duan, M.; Pappas, E.; Fritsch, B.; Boyle, R.; Gupta, S.; Stalley, P.; Horsley, M.; Steffens, D. The effectiveness of robotic hip and knee arthroplasty on patient-reported outcomes: A systematic review and meta-analysis. Int. Orthop. 2018, 43, 1283–1295. [Google Scholar] [CrossRef]
- Samuel, L.T.; Acuña, A.J.; Mahmood, B.; Emara, A.K.; Kamath, A.F. Comparing early and mid-term outcomes between robotic-arm assisted and manual total hip arthroplasty: A systematic review. J. Robot. Surg. 2021, 16, 735–748. [Google Scholar] [CrossRef]
- Remily, E.A.; Nabet, A.; Sax, O.C.; Douglas, S.J.; Pervaiz, S.S.; Delanois, R.E. Impact of Robotic Assisted Surgery on Outcomes in Total Hip Arthroplasty. Arthroplast. Today 2021, 9, 46. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, J.; Wang, P.; Zhu, S.; Qi, W.; Peng, H.; Yu, L.; Qian, W. Robotic-assisted compared with conventional total hip arthroplasty: Systematic review and meta-analysis. Postgrad. Med. J. 2018, 94, 1112. [Google Scholar] [CrossRef]
- Kayani, B.; Konan, S.; Huq, S.S.; Ibrahim, M.S.; Ayuob, A.; Haddad, F.S. The learning curve of robotic-arm assisted acetabular cup positioning during total hip arthroplasty. HIP Int. 2021, 31, 311–319. [Google Scholar] [CrossRef]
- Bendich, I.; Vigdorchik, J.M.; Sharma, A.K.; Mayman, D.J.; Sculco, P.K.; Anderson, C.; Della Valle, A.G.; Su, E.P.; Jerabek, S.A. Robotic Assistance for Posterior Approach Total Hip Arthroplasty Is Associated With Lower Risk of Revision for Dislocation When Compared to Manual Techniques. J. Arthroplast. 2022, 37, 1124–1129. [Google Scholar] [CrossRef]
- Shaw, J.H.; Rahman, T.M.; Wesemann, L.D.; Jiang, C.Z.; Lindsay-Rivera, K.G.; Davis, J.J. Comparison of Postoperative Instability and Acetabular Cup Positioning in Robotic-Assisted Versus Traditional Total Hip Arthroplasty. J. Arthroplast. 2022, 37, S881–S889. [Google Scholar] [CrossRef]
- Maldonado, D.R.; Go, C.C.; Kyin, C.; Rosinsky, P.J.; Shapira, J.; Lall, A.C.; Domb, B.G. Robotic Arm-assisted Total Hip Arthroplasty is More Cost-Effective Than Manual Total Hip Arthroplasty: A Markov Model Analysis. J. Am. Acad. Orthop. Surg. 2021, 29, e168–e177. [Google Scholar] [CrossRef]
- Kirchner, G.J.; Lieber, A.M.; Haislup, B.; Kerbel, Y.E.; Moretti, V.M. The Cost of Robot-assisted Total Hip Arthroplasty: Comparing Safety and Hospital Charges to Conventional Total Hip Arthroplasty. J. Am. Acad. Orthop. Surg. 2021, 29, 609–615. [Google Scholar] [CrossRef]
- Fontalis, A.; Kayani, B.; Asokan, A.; Haddad, I.C.; Tahmassebi, J.; Konan, S.; Oussedik, S.; Haddad, F.S. Inflammatory Response in Robotic-Arm-Assisted Versus Conventional Jig-Based TKA and the Correlation with Early Functional Outcomes: Results of a Prospective Randomized Controlled Trial. J. Bone Jt. Surg. 2022, 104, 1905–1914. [Google Scholar] [CrossRef]
- Fontalis, A.; Epinette, J.A.; Thaler, M.; Zagra, L.; Khanduja, V.; Haddad, F.S. Advances and innovations in total hip arthroplasty. SICOT J. 2021, 7, 26. [Google Scholar] [CrossRef]
- Eckhard, L.; Munir, S.; Wood, D.; Talbot, S.; Brighton, R.; Walter, B.; Baré, J. The ceiling effects of patient reported outcome measures for total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2021, 107, 102758. [Google Scholar] [CrossRef]













Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogilvie, A.; Kim, W.J.; Asirvatham, R.D.; Fontalis, A.; Putzeys, P.; Haddad, F.S. Robotic-Arm-Assisted Total Hip Arthroplasty: A Review of the Workflow, Outcomes and Its Role in Addressing the Challenge of Spinopelvic Imbalance. Medicina 2022, 58, 1616. https://doi.org/10.3390/medicina58111616
Ogilvie A, Kim WJ, Asirvatham RD, Fontalis A, Putzeys P, Haddad FS. Robotic-Arm-Assisted Total Hip Arthroplasty: A Review of the Workflow, Outcomes and Its Role in Addressing the Challenge of Spinopelvic Imbalance. Medicina. 2022; 58(11):1616. https://doi.org/10.3390/medicina58111616
Chicago/Turabian StyleOgilvie, Andrew, Woo Jae Kim, Rhody David Asirvatham, Andreas Fontalis, Pierre Putzeys, and Fares S. Haddad. 2022. "Robotic-Arm-Assisted Total Hip Arthroplasty: A Review of the Workflow, Outcomes and Its Role in Addressing the Challenge of Spinopelvic Imbalance" Medicina 58, no. 11: 1616. https://doi.org/10.3390/medicina58111616
APA StyleOgilvie, A., Kim, W. J., Asirvatham, R. D., Fontalis, A., Putzeys, P., & Haddad, F. S. (2022). Robotic-Arm-Assisted Total Hip Arthroplasty: A Review of the Workflow, Outcomes and Its Role in Addressing the Challenge of Spinopelvic Imbalance. Medicina, 58(11), 1616. https://doi.org/10.3390/medicina58111616







