Effects of Photobiomodulation in Patients Presenting with Reticular Pseudodrusen: A Retrospective Observational Case Series Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Treatment and Imaging Modalities
2.3. Descriptive Analysis
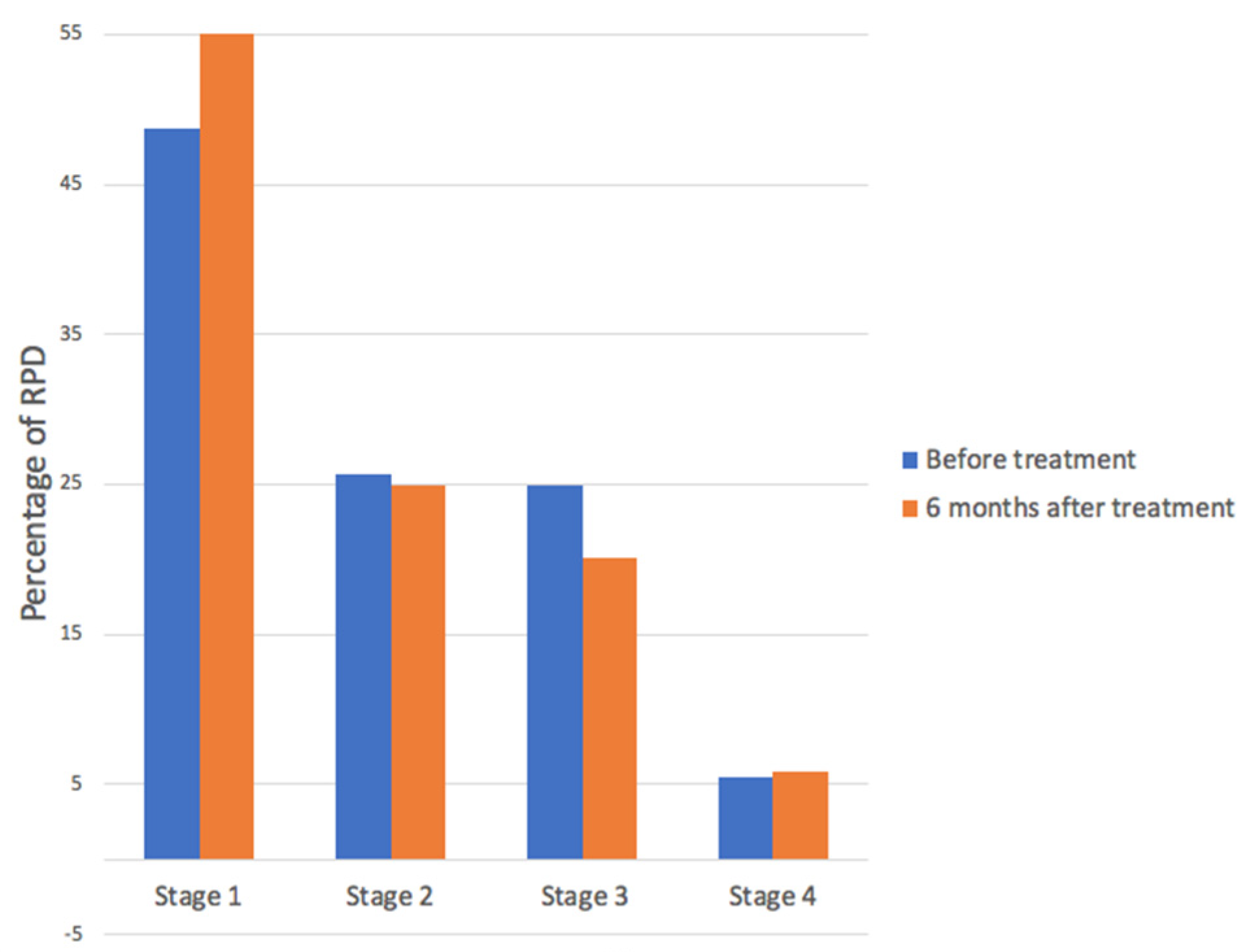
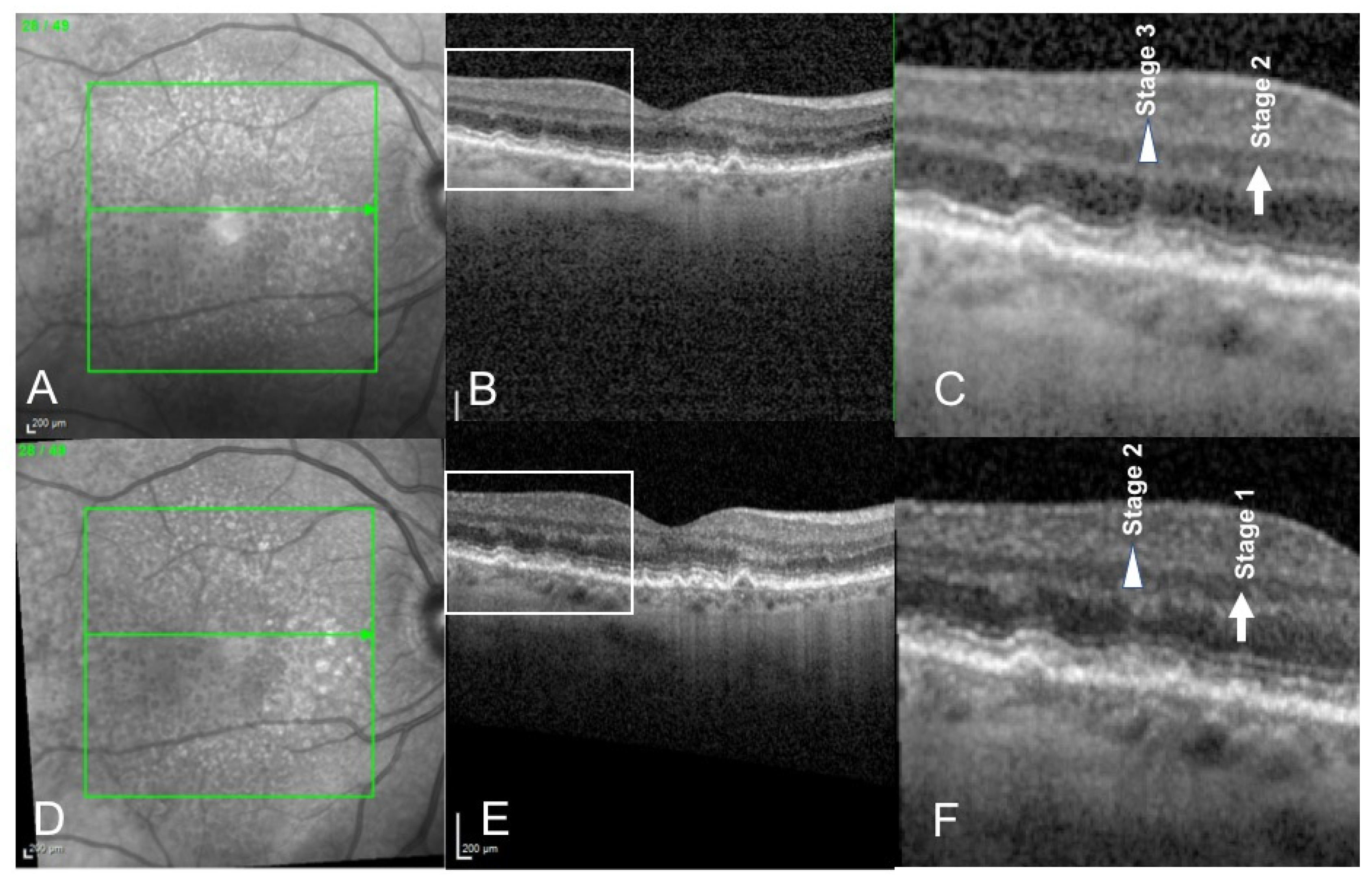
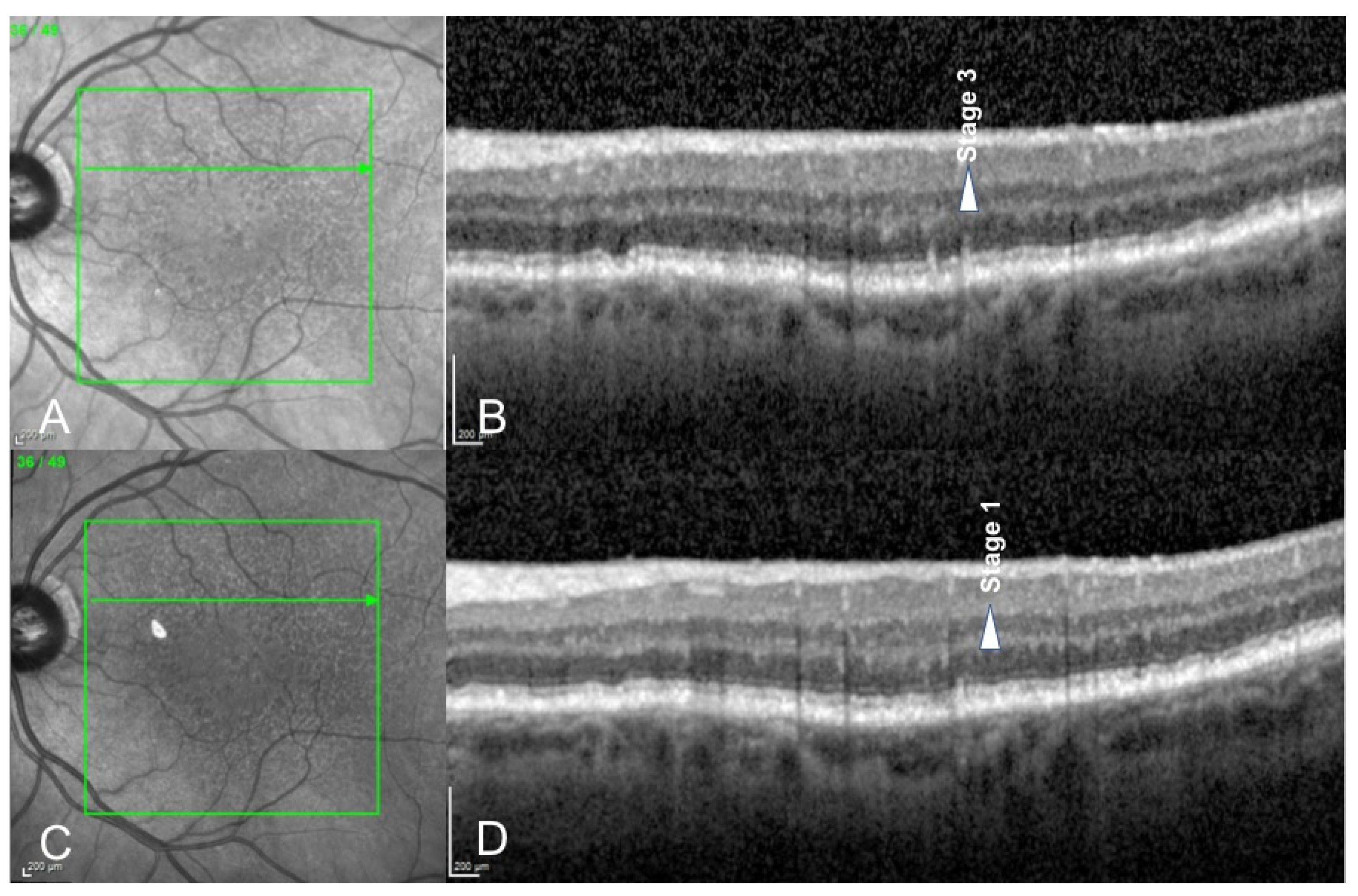
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coleman, H.R.; Chan, C.C.; Ferris, F.L.; Chew, E.Y. Age-related macular degeneration. Lancet 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, U.; Peto, T. Current Perspective on Age-Related Macular Degeneration. JAMA 2020, 324, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Rodrigues, G.A. Progress and perspectives on the role of RPE cell inflammatory responses in the development of age-related macular degeneration. J. Inflamm. Res. 2008, 1, 49–65. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Khandhadia, S.; Cherry, J.; Lotery, A.J. Age-Related Macular Degeneration. In Neurodegenerative Diseases; Advances in Experimental Medicine and Biology; Ahmad, S.I., Ed.; Springer: New York, NY, USA, 2012; Volume 724, pp. 15–36. [Google Scholar] [CrossRef]
- Davis, M.D.; Gangnon, R.E.; Lee, L.Y.; Hubbard, L.D.; Klein, B.E.; Klein, R.; Ferris, F.L.; Bressler, S.B.; Milton, R.C. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch. Ophthalmol. 2005, 123, 1484–1498. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical Classification of Age-related Macular Degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef]
- Mimoun, G.; Soubrane, G.; Coscas, G. Macular drusen. J. Fr. Ophtalmol. 1990, 13, 511–530. [Google Scholar] [PubMed]
- Yoneyama, S.; Sakurada, Y.; Mabuchi, F.; Imasawa, M.; Sugiyama, A.; Kubota, T.; Iijima, H. Genetic and clinical factors associated with reticular pseudodrusen in exudative age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Dubois, L.; Tadayoni, R.; Delahaye-Mazza, C.; Debibie, C.; Quentel, G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularization. Br. J. Ophthalmol. 2007, 91, 354–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliem, M.; Hendig, D.; Finger, R.P.; Holz, F.G.; Charbel Issa, P. Reticular Pseudodrusen Associated with a Diseased Bruch Membrane in Pseudoxanthoma Elasticum. JAMA Ophthalmol. 2015, 133, 581–588. [Google Scholar] [CrossRef]
- Gliem, M.; Müller, P.L.; Mangold, E.; Bolz, H.J.; Stöhr, H.; Weber, B.H.; Holz, F.G.; Issa, P.C. Reticular Pseudodrusen in Sorsby Fundus Dystrophy. Ophthalmology 2015, 122, 1555–1562. [Google Scholar] [CrossRef]
- Chan, H.; Cougnard-Grégoire, A.; Delyfer, M.-N.; Combillet, F.; Rougier, M.-B.; Schweitzer, C.; Dartigues, J.-F.; Korobelnik, J.-F.; Delcourt, C. Multimodal Imaging of Reticular Pseudodrusen in a Population-Based Setting: The Alienor Study. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3058. [Google Scholar] [CrossRef] [Green Version]
- Ueda-Arakawa, N.; Ooto, S.; Nakata, I.; Yamashiro, K.; Tsujikawa, A.; Oishi, A.; Yoshimura, N. Prevalence and Genomic Association of Reticular Pseudodrusen in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2012, 155, 260–269.e2. [Google Scholar] [CrossRef]
- Finger, R.P.; Chong, E.; McGuinness, M.B.; Robman, L.D.; Aung, K.Z.; Giles, G.; Baird, P.N.; Guymer, R. Reticular Pseudodrusen and Their Association with Age-Related Macular Degeneration: The Melbourne Collaborative Cohort Study. Ophthalmology 2015, 123, 599–608. [Google Scholar] [CrossRef]
- Wu, Z.; Fletcher, E.L.; Kumar, H.; Greferath, U.; Guymer, R.H. Reticular pseudodrusen: A critical phenotype in age-related macular degeneration. Prog. Retin. Eye Res. 2021, 88, 101017. [Google Scholar] [CrossRef]
- Querques, G.; Massamba, N.; Srour, M.; Boulanger, E.; Georges, A.; Souied, E.H. Impact of reticular pseudodrusen on macular function. Retina 2014, 34, 321–329. [Google Scholar] [CrossRef]
- Querques, G.; Sacconi, R.; Gelormini, F.; Borrelli, E.; Prascina, F.; Zucchiatti, I.; Querques, L.; Bandello, F. Subthreshold laser treatment for reticular pseudodrusen secondary to age-related macular degeneration. Sci. Rep. 2021, 11, 2193. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lima, F.; Rojas, J.C. Low-level light therapy of the eye and brain. Eye Brain 2011, 3, 49–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, J.; Wood, J.P.; Chidlow, G.; Gillies, M.C.; Casson, R.J. Retinal pigment epithelium in the pathogenesis of age-related macular degeneration and photobiomodulation as a potential therapy? Clin. Exp. Ophthalmol. 2018, 46, 670–686. [Google Scholar] [CrossRef] [Green Version]
- Geneva, I.I. Photobiomodulation for the treatment of retinal diseases: A review. Int. J. Ophthalmol. 2016, 9, 145–152. [Google Scholar] [CrossRef]
- Albarracin, R.; Eells, J.; Valter, K. Photobiomodulation Protects the Retina from Light-Induced Photoreceptor Degeneration. Investig. Opthalmology Vis. Sci. 2011, 52, 3582–3592. [Google Scholar] [CrossRef] [Green Version]
- Eells, J.T.; Wong-Riley, M.T.; Verhoeve, J.; Henry, M.; Buchman, E.V.; Kane, M.P.; Gould, L.J.; Das, R.; Jett, M.; Hodgson, B. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion 2004, 4, 559–567. [Google Scholar] [CrossRef]
- Tang, J.; Du, Y.; Lee, C.A.; Talahalli, R.; Eells, J.T.; Kern, T.S. Low-Intensity Far-Red Light Inhibits Early Lesions That Contribute to Diabetic Retinopathy: In Vivo and In Vitro. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3681–3690. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, S.N.; Devenyi, R.G.; Munk, M.R.; Croissant, C.L.; Tedford, S.E.; Rückert, R.; Walker, M.G.; Patino, B.E.; Chen, L.; Nido, M.; et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina 2020, 40, 1471–1482. [Google Scholar] [CrossRef]
- Merry, G.F.; Munk, M.R.; Dotson, R.S.; Walker, M.G.; Devenyi, R.G. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2016, 95, e270–e277. [Google Scholar] [CrossRef] [Green Version]
- Querques, G.; Canoui-Poitrine, F.; Coscas, F.; Massamba, N.; Querques, L.; Mimoun, G.; Bandello, F.; Souied, E.H. Analysis of Progression of Reticular Pseudodrusen by Spectral Domain–Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Eells, J.T.; Gopalakrishnan, S.; Valter, K. Near-Infrared Photobiomodulation in Retinal Injury and Disease. In Retinal Degenerative Diseases; Springer: Cham, Switzerland, 2015; pp. 437–441. [Google Scholar] [CrossRef]
- Ivandic, B.T.; Ivandic, T. Low-Level Laser Therapy Improves Vision in Patients with Age-Related Macular Degeneration. Photomed. Laser Surg. 2008, 26, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Merry, G.; Dotson, R.; Devenyi, R.; Markowitz, S.; Reyes, S. Photobiomodulation as a New Treatment for Dry Age Related Macular Degeneration. Results from the Toronto and Oak Ridge Photobimodulation Study in AMD (TORPA). Investig. Ophthalmol. Vis. Sci. 2012, 53, 2049. [Google Scholar]
- Grewal, M.K.; Sivapathasuntharam, C.; Chandra, S.; Gurudas, S.; Chong, V.; Bird, A.; Jeffery, G.; Sivaprasad, S. A Pilot Study Evaluating the Effects of 670 nm Photobiomodulation in Healthy Ageing and Age-Related Macular Degeneration. J. Clin. Med. 2020, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, H.M.; Mehanna, C.-J.; De Rosa, I.; Miere, A.; Souied, E. Effects of Photobiomodulation in Patients Presenting with Reticular Pseudodrusen: A Retrospective Observational Case Series Study. Medicina 2022, 58, 1662. https://doi.org/10.3390/medicina58111662
Le HM, Mehanna C-J, De Rosa I, Miere A, Souied E. Effects of Photobiomodulation in Patients Presenting with Reticular Pseudodrusen: A Retrospective Observational Case Series Study. Medicina. 2022; 58(11):1662. https://doi.org/10.3390/medicina58111662
Chicago/Turabian StyleLe, Hoang Mai, Carl-Joe Mehanna, Irene De Rosa, Alexandra Miere, and Eric Souied. 2022. "Effects of Photobiomodulation in Patients Presenting with Reticular Pseudodrusen: A Retrospective Observational Case Series Study" Medicina 58, no. 11: 1662. https://doi.org/10.3390/medicina58111662




