Concurrent Aerobic Plus Resistance Training Elicits Different Effects on Short-Term Blood Pressure Variability of Hypertensive Patients in Relation to Their Nocturnal Blood Pressure Pattern
Abstract
1. Introduction
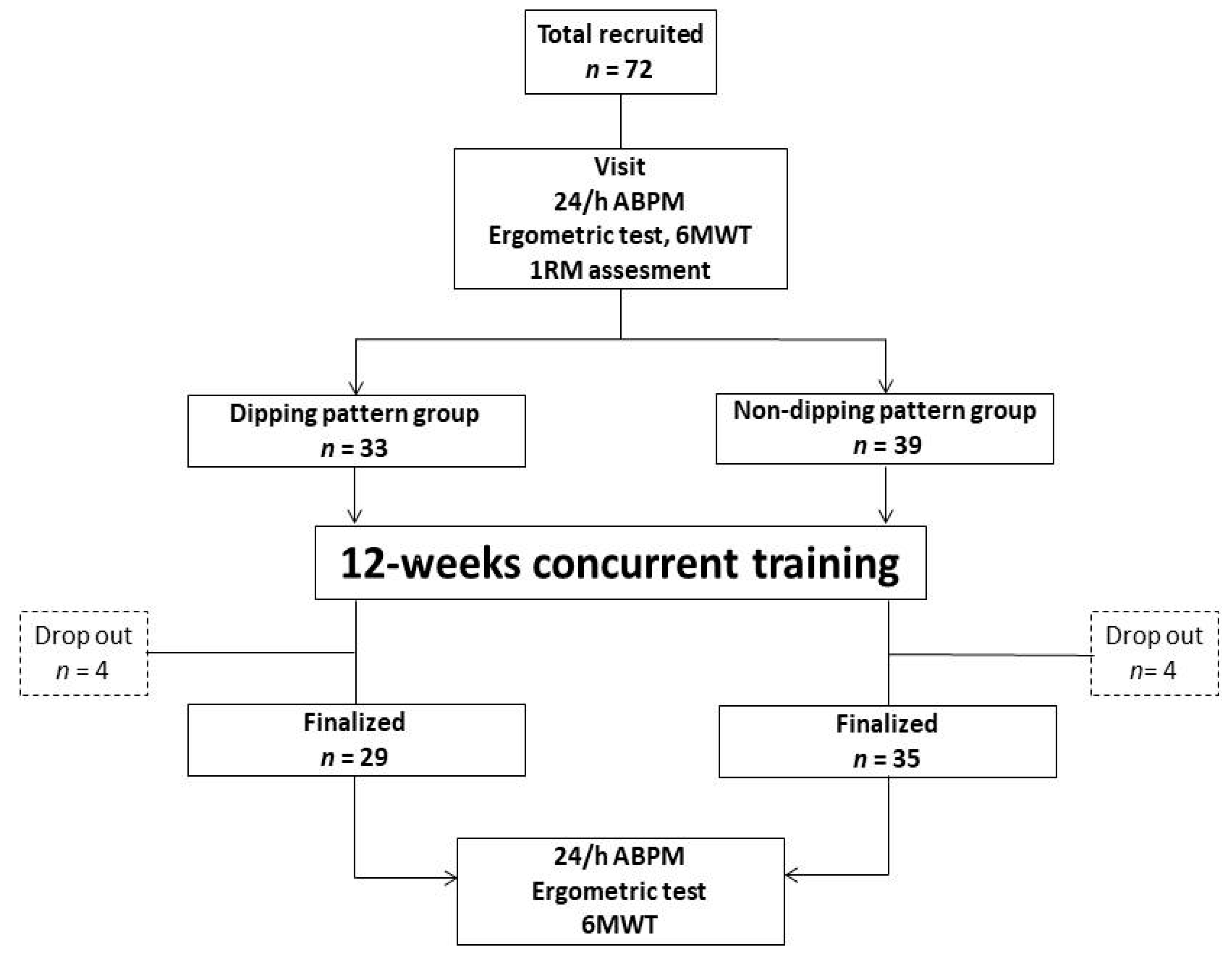
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parati, G.; Ochoa, J.E.; Lombardi, C.; Bilo, G. Assessment and management of blood-pressure variability. Nat. Rev. Cardiol. 2013, 10, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Cremer, A.; Doublet, J.; Boulestreau, R.; Gaudissard, J.; Tzourio, C.; Gosse, P. Short-term blood pressure variability, arterial stiffness, and cardiovascular events: Results from the Bordeaux cohort. J. Hypertens. 2021, 39, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, X.; Jia, H.; Yu, B. The Effect of Blood Pressure Variability on Coronary Atherosclerosis Plaques. Front. Cardiovasc. Med. 2022, 15, 803810. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA. Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults. JACC 2018, 71, e127–e248. [Google Scholar] [CrossRef]
- Caminiti, G.; Mancuso, A.; Raposo, A.F.; Fossati, C.; Selli, S.; Volterrani, M. Different exercise modalities exert opposite acute effects on short-term blood pressure variability in male patients with hypertension. Eur. J. Prev. Cardiol. 2019, 26, 1028–1031. [Google Scholar] [CrossRef]
- Smolensky, M.H.; Hermida, R.C.; Castriotta, R.J.; Portaluppi, F. Role of sleep-wakecycle on blood pressure circadian rhythms and hypertension. Sleep Med. 2007, 8, 668–680. [Google Scholar] [CrossRef]
- Ingelsson, E.; Björklund-Bodegård, K.; Lind, L.; Arnlöv, J.; Sundström, J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA 2006, 295, 2859–2866. [Google Scholar] [CrossRef]
- Kario, K.; Pickering, T.G.; Matsuo, T.; Hoshide, S.; Schwartz, J.E.; Shimada, K. Strokeprognosis and abnormal nocturnalblood pressure falls in older hypertensives. Hypertension 2001, 38, 852–857. [Google Scholar] [CrossRef]
- ABC-H Investigators Roush, G.C.; Fagard, R.H.; Salles, G.F.; Pierdomenico, S.D.; Reboldi, G.; Verdecchia, P.; Eguchi, K.; Kario, K.; Hoshide, S.; Polonia, J.; et al. Prognostic impact from clinic, daytime, and night-time systolic blood pressure in nine cohorts of 13,844 patients with hypertension. J. Hypertens. 2014, 32, 2332–2340. [Google Scholar] [CrossRef]
- Fagard, R.H.; Celis, H.; Thijs, L.; Staessen, J.A.; Clement, D.L.; De Buyzere, M.L.; De Bacquer, D.A. Daytime and nighttime blood pressure as predictors of death and cause-specific cardiovascular events in hypertension. Hypertension 2008, 51, 55–61. [Google Scholar] [CrossRef]
- Ohkubo, T.; Hozawa, A.; Yamaguchi, J.; Kikuya, M.; Ohmori, K.; Michimata, M.; Matsubara, M.; Hashimoto, J.; Hoshi, H.; Araki, T.; et al. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: The Ohasama study. J. Hypertens. 2002, 20, 2183–2189. [Google Scholar] [CrossRef] [PubMed]
- Salles, G.F.; Reboldi, G.; Fagard, R.H.; Cardoso, C.R.; Pierdomenico, S.D.; Verdecchia, P.; Roush, G.C. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: The ambulatory blood pressure collaboration in patients with hypertension (ABC-H) meta-analysis. Hypertension 2016, 67, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Palatini, P.; Reboldi, G.; Saladini, F.; Angeli, F.; Mos, L.; Rattazzi, M.; Vriz, O.; Verdecchia, P. Dipping pattern and short-term blood pressure variability are stronger predictors of cardiovascular events than average 24-h blood pressure in young hypertensive subjects. Eur. J. Prev. Cardiol. 2022, 5, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, S.; Garofalo, C.; Gabbai, F.B.; Chiodini, P.; Signoriello, S.; Paoletti, E.; Ravera, M.; Bussalino, E.; Bellizzi, V.; Liberti, M.E.; et al. Dipping Status, Ambulatory Blood Pressure Control, Cardiovascular Disease, and Kidney Disease Progression: A Multicenter Cohort Study of CKD. Am. J. Kidney Dis. 2022. Corrected proof, published online, In press. [Google Scholar] [CrossRef]
- Rimoldi, S.F.; Scherrer, U.; Messerli, F.H. Secondary arterial hypertension: When, who, and how to screen? Eur. Heart J. 2014, 35, 1245–1254. [Google Scholar] [CrossRef]
- Mezue, K.; Isiguzo, G.; Madu, C.; Nwuruku, G.; Rangaswami, J.; Baugh, D.; Madu, E. Nocturnal Non-dipping Blood Pressure Profile in Black Normotensives Is Associated with Cardiac Target Organ Damage. Ethn. Dis. 2016, 26, 279–284. [Google Scholar] [CrossRef]
- Nami, R.; Mondillo, S.; Agricola, E.; Lenti, S.; Ferro, G.; Nami, N.; Tarantino, M.; Glauco, G.; Spanò, E.; Gennari, C. Aerobicexercise training fails to reduce blood pressure in nondipper-typehypertension. Am. J. Hypertens. 2000, 13, 593–600. [Google Scholar] [CrossRef]
- Boa Sorte Silva, N.C.; Gregory, M.A.; Gill, D.P.; McGowan, C.L.; Petrella, R.J. The Impact of Blood Pressure Dipping Status on Cognition, Mobility, and Cardiovascular Health in Older Adults Following an Exercise Program. Gerontol. Geriatr. Med. 2018, 4, 2333721418770333. [Google Scholar] [CrossRef]
- Brooks, D.; Solway, S.; Gibbons, W.J. ATS statement on six-minute walk test. Am. J. Respir. Crit. Care Med. 2003, 167, 1287. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Mena, L.; Pintos, S.; Queipo, N.V.; Aizpúrua, J.A.; Maestre, G.; Sulbarán, T. A reliable index for the prognostic significance of blood pressure variability. J. Hypertens. 2005, 23, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.E.; Weir, J.P. ASEP Procedures Recommendation I: Accurate Assessment of Muscular Strength and Power. J. Exerc. Physiol. 2001, 4, 1–21. [Google Scholar]
- Pagonas, N.; Dimeo, F.; Bauer, F.; Seibert, F.; Kiziler, F.; Zidek, W.; Westhoff, T.H. The impact of aerobic exercise on blood pressurevariability. J. Hum. Hypertens. 2014, 28, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, G.; Iellamo, F.; Mancuso, A.; Cerrito, A.; Montano, M.; Manzi, V.; Volterrani, M. Effects of 12 weeks of aerobic versus combined aerobic plus resistance exercise training on short-term blood pressure variability in patients with hypertension. J. Appl. Physiol. 2021, 130, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Matias, L.A.S.; Mariano, I.M.; Batista, J.P.; de Souza, T.C.F.; Amaral, A.L.; Dechichi, J.G.C.; de Lima Rodrigues, M.; Carrijo, V.H.V.; Cunha, T.M.; Puga, G.M. Acute and chronic effects of combined exercise on ambulatory blood pressure and its variability in hypertensive postmenopausal women. Chin. J. Physiol. 2020, 63, 227–234. [Google Scholar]
- Routledge, F.S.; McFetridge-Durdle, J.A.; Dean, C.R. Stress, menopausal status and nocturnal blood pressure dipping patterns among hypertensive women. Can. J. Cardiol. 2009, 25, e157–e163. [Google Scholar] [CrossRef]
- Mitu, O.; Roca, M.; Gurzu, B.; Magdalena, M.; Constantin, L.; Jitaru, A.; Al Namat, R.; Gavril, R.S.; Mitu, F. Predictors of the blood pressure non-dipping profile innewly diagnosed hypertensive patients. J. Hypertens. Res. 2016, 2, 121. [Google Scholar]
- Ramirez-Jimenez, M.; Morales-Palomo, F.; Moreno-Cabañas, A.; Alvarez-Jimenez, L.; Ortega, J.F.; Mora-Rodriguez, R. Aerobic exercise training improves nocturnal blood pressure dipping in medicated hypertensive individuals. Blood Press. Monit. 2022, 27, 272–275. [Google Scholar] [CrossRef]
- Takeda, A.; Toda, T.; Fujii, T.; Matsui, N. Bedtime administration of long-acting antihypertensive drugs restores normal nocturnal blood pressure fall in nondippers with essential hypertension. Clin. Exp. Nephrol. 2009, 13, 467–472. [Google Scholar] [CrossRef]
- Park, S.; Jastremski, A.C.; Wallace, J.P. Time of day for exercise on blood pressure reduction in dipping and nondipping hypertension. J. Human Hypertens. 2005, 19, 597–605. [Google Scholar] [CrossRef][Green Version]
- Okamoto, L.E.; Gamboa, A.; Shibao, C.; Black, B.K.; Diedrich, A.; Raj, S.R.; Robertson, D.; Biaggioni, I. Nocturnal blood pressure dipping in the hypertension of autonomic failure. Hypertension 2009, 53, 363–369. [Google Scholar] [CrossRef]
- de la Sierra, A.; Redon, J.; Banegas, J.R.; Segura, J.; Parati, G.; Gorostidi, M.; de la Cruz, J.J.; Sobrino, J.; Llisterri, J.L.; Alonso, J.; et al. Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension 2009, 53, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Routledge, F.; McFetridge-Durdle, J. Nondipping blood pressure patterns among individuals with essential hypertension: A review of the literature. Eur. J. Cardiovasc. Nurs. 2007, 6, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Mousa, T.; el-Sayed, M.A.; Motawea, A.K.; Salama, M.A.; Elhendy, A. Association of blunted nighttime blood pressure dipping with coronary artery stenosis in men. Am. J. Hypertens. 2004, 17, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Sun, L.; Gao, Y.; Guo, Q.; Guo, L.; Wang, X.; Wang, G. Blood pressure reverse dipping may associate with stable coronary artery disease in patients with essential hypertension: A cross-sectional study. Sci. Rep. 2016, 6, 25410. [Google Scholar] [CrossRef] [PubMed]
- Tsioufis, C.; Andrikou, I.; Thomopoulos, C.; Syrseloudis, D.; Stergiou, G.; Stefanadis, C. Increased nighttime blood pressure or nondipping profile for prediction of cardiovascular outcomes. J. Hum. Hypertens. 2011, 25, 281–293. [Google Scholar] [CrossRef]
| Overall Population (n = 64) | Non-Dipper (n = 35) | Dipper (n = 29) | |
|---|---|---|---|
| Age, y Males/females, n (%) | 66.1 ± 12.7 46 (71.8)/18 (28.2) | 68.6 ± 13.4 * 22 (62.8)/13 (37.2) | 62.7 ± 15.2 24 (82.7)/5(17.2) |
| BMI, Kg/m2 | 27.6 ± 7.1 | 28.4 ± 8.3 * | 26.2 ± 7.8 |
| Resting HR, bpm | 68.7 ± 13.4 | 68.9 ± 11.1 | 69.0 ± 17.2 |
| Office systolic BP, mmHg | 123.3 ± 44.5 | 122.9 ± 39.2 | 123.7 ± 34.2 |
| Office diastolic BP, mmHg | 83.5 ± 11.8 | 84.6 ± 16.3 | 82.0 ± 14.1 |
| Previous CABG/PCI | 23 (35.9)/48 (75.0) | 14 (40.0)/27 (77.1) | 9 (31.1)/21 (72.4) |
| Comorbidities | |||
| Diabetes, n (%) | 11 (17.1) | 6 (17.1) | 5 (17.2) |
| COPD, n (%) | 19 (29.6) | 11 (34.4) | 8 (27.5) |
| OSAS, n (%) | 15 (23.4) | 11 (31.4) * | 4 (13.7) |
| Carotid artery disease, n (%) | 28 (43.7) | 15 (42.8) | 13 (44.8) |
| History of smoke, n (%) | 39 (60.9) | 21 (60.0) | 18 (62.0) |
| Anti-hypertensive treatment Anti-hypertensive drugs, n ACE-i/ARBs, n (%) Calcium-channel antagonists, n (%) Beta-blockers, n (%) Thiazide diuretics, n (%) Aldosteron-antagonists, n (%) Clonidine, n (%) Nitrates, n (%) Aliskiren, n (%) | 3.4 ± 1.1 58 (90.6) 39 (60.9) 28 (43.7) 46 (71.8) 15 (23.4) 7 (10.9) 21 (32.8) 3 (4.6) | 4.2 ± 1.7 * 31 (88.5) 22 (66.8) 18 (51.4) 25 (71.4) 8 (22.8) 5 (14.2) 13 (37.1) 3 (8.5) | 2.5 ± 0.9 27(93.1) 17 (58.6) 10 (34.4) 21 (72.4) 7 (24.1) 2 (6.8) 8 (27.5) 0 |
| Non-Dipping Pattern | Dipping Pattern | |||
|---|---|---|---|---|
| Baseline | 12-Weeks | Baseline | 12-Weeks | |
| Exercise tolerance | ||||
| Time at ergometric test, s | 413.2 ± 44 | 567.2 ± 44 ⴕ | 407.8 ± 44 | 571.5 ± 39 ⴕ |
| Distance at 6MWT, m | 395.6 ± 65 | 449.3 ± 71 ⴕ | 378.6 ± 555 | 439.9 ± 70 ⴕ |
| Borg’s scale | 10.8 ± 1.7 | 7.9 ± 1.6 ⴕ | 10.2 ± 1.9 | 7.3 ± 1.1 ⴕ |
| Blood Pressure | ||||
| 24/h SBP, mmHg | 121.6 ± 28.5 | 120.4 ± 32.4 | 123.7 ± 36.2 | 115.3 ± 29.7 ⴕ * |
| Daytime SBP, mmHg | 124.3 ± 30.6 | 123.6 ± 34.0 | 130.6 ± 28.5 | 122.1 ± 31.3 ⴕ * |
| Nighttime SBP, mmHg | 118.8 ± 123.3 | 116.8 ± 26.1 | 111.7 ± 22.3 | 110.0 ± 24.8 |
| 24/h DBP, mmHg | 70.3 ± 16.3 | 69.3 ± 18.1 | 70.8 ± 15.4 | 69.4 ± 19.4 |
| Daytime DBP, mmHg | 73.4 ± 22.0 | 73.3 ± 18.4 | 77.3 ± 22.3 | 75.8 ± 17.9 |
| Nighttime DBP, mmHg | 67.1 ± 20.5 | 64.9 ± 17.5 | 65.7 ± 19.6 | 63.0 ± 23.6 |
| BP Variability | ||||
| 24/h SBPV, mmHg | 8.8 ± 2.0 | 8.6 ± 1.7 | 9.3 ± 2.1 | 8.1 ± 1.8 ⴕ * |
| Daytime SBPV, mmHg | 8.5 ± 1.6 | 8.4 ± 1.2 | 9.4 ± 2.2 | 8.0 ± 2.0 ⴕ * |
| Nighttime SBPV, mmHg | 9.4 ± 2.4 | 9.2 ± 2.0 | 9.4 ± 1.7 | 9.2 ± 1.9 |
| 24/h DBPV, mmHg | 6.9 ± 1.1 | 6.7 ± 0.8 | 6.5 ± 1.3 | 6.3 ± 1.5 |
| Daytime DBPV, mmHg | 6.7 ± 0.7 | 6.5 ± 1.2 | 6.6 ± 0.7 | 6.2 ± 0.9 |
| Nighttime DBPV, mmHg | 7.1 ± 1.9 | 7.5 ± 1.3 | 6.5 ± 0.8 | 6.7 ± 0.6 |
| Heart rate | ||||
| 24/h, bpm | 63.8 ± 25.6 | 63.1 ± 23.2 | 61.1 ± 18.5 | 60.5 ± 27.8 |
| Daytime, bpm | 65.2 ± 21.2 | 64.6 ± 19.4 | 62.9 ± 19.7 | 61.4 ± 22.6 |
| Nighttime, bpm | 62.5 ± 28.3 | 61.8 ± 21.1 | 58.6 ± 26.2 | 59.7 ± 19.9 |
| F | p | |
|---|---|---|
| Age | 1.44 | 0.14 |
| BMI | 2.31 | 0.08 |
| OSAS | 0.46 | 0.19 |
| Nocturnal BP pattern | 5.36 | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caminiti, G.; Iellamo, F.; Perrone, M.A.; Marazzi, G.; Gismondi, A.; Cerrito, A.; Franchini, A.; Volterrani, M. Concurrent Aerobic Plus Resistance Training Elicits Different Effects on Short-Term Blood Pressure Variability of Hypertensive Patients in Relation to Their Nocturnal Blood Pressure Pattern. Medicina 2022, 58, 1682. https://doi.org/10.3390/medicina58111682
Caminiti G, Iellamo F, Perrone MA, Marazzi G, Gismondi A, Cerrito A, Franchini A, Volterrani M. Concurrent Aerobic Plus Resistance Training Elicits Different Effects on Short-Term Blood Pressure Variability of Hypertensive Patients in Relation to Their Nocturnal Blood Pressure Pattern. Medicina. 2022; 58(11):1682. https://doi.org/10.3390/medicina58111682
Chicago/Turabian StyleCaminiti, Giuseppe, Ferdinando Iellamo, Marco Alfonso Perrone, Giuseppe Marazzi, Alessandro Gismondi, Anna Cerrito, Alessio Franchini, and Maurizio Volterrani. 2022. "Concurrent Aerobic Plus Resistance Training Elicits Different Effects on Short-Term Blood Pressure Variability of Hypertensive Patients in Relation to Their Nocturnal Blood Pressure Pattern" Medicina 58, no. 11: 1682. https://doi.org/10.3390/medicina58111682
APA StyleCaminiti, G., Iellamo, F., Perrone, M. A., Marazzi, G., Gismondi, A., Cerrito, A., Franchini, A., & Volterrani, M. (2022). Concurrent Aerobic Plus Resistance Training Elicits Different Effects on Short-Term Blood Pressure Variability of Hypertensive Patients in Relation to Their Nocturnal Blood Pressure Pattern. Medicina, 58(11), 1682. https://doi.org/10.3390/medicina58111682









