Organic Bone Matrix Component Type I and V Collagen Are Not Destructed in Bisphosphonate-Associated Osteonecrosis of the Jaws
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Sample Obtainment
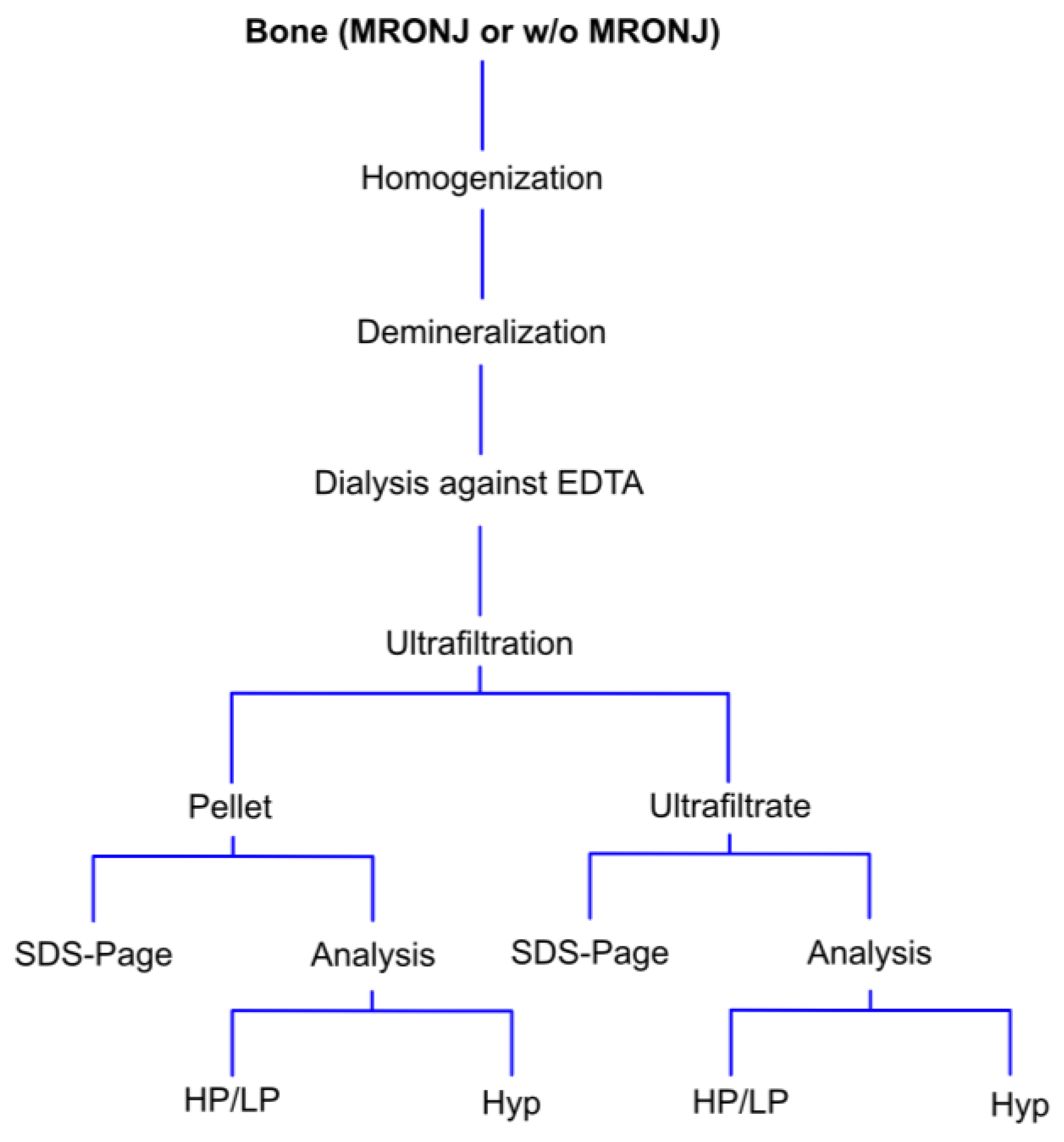
2.2. Separatin of Broken Collagen Fraction from Intact Collagen Molecules
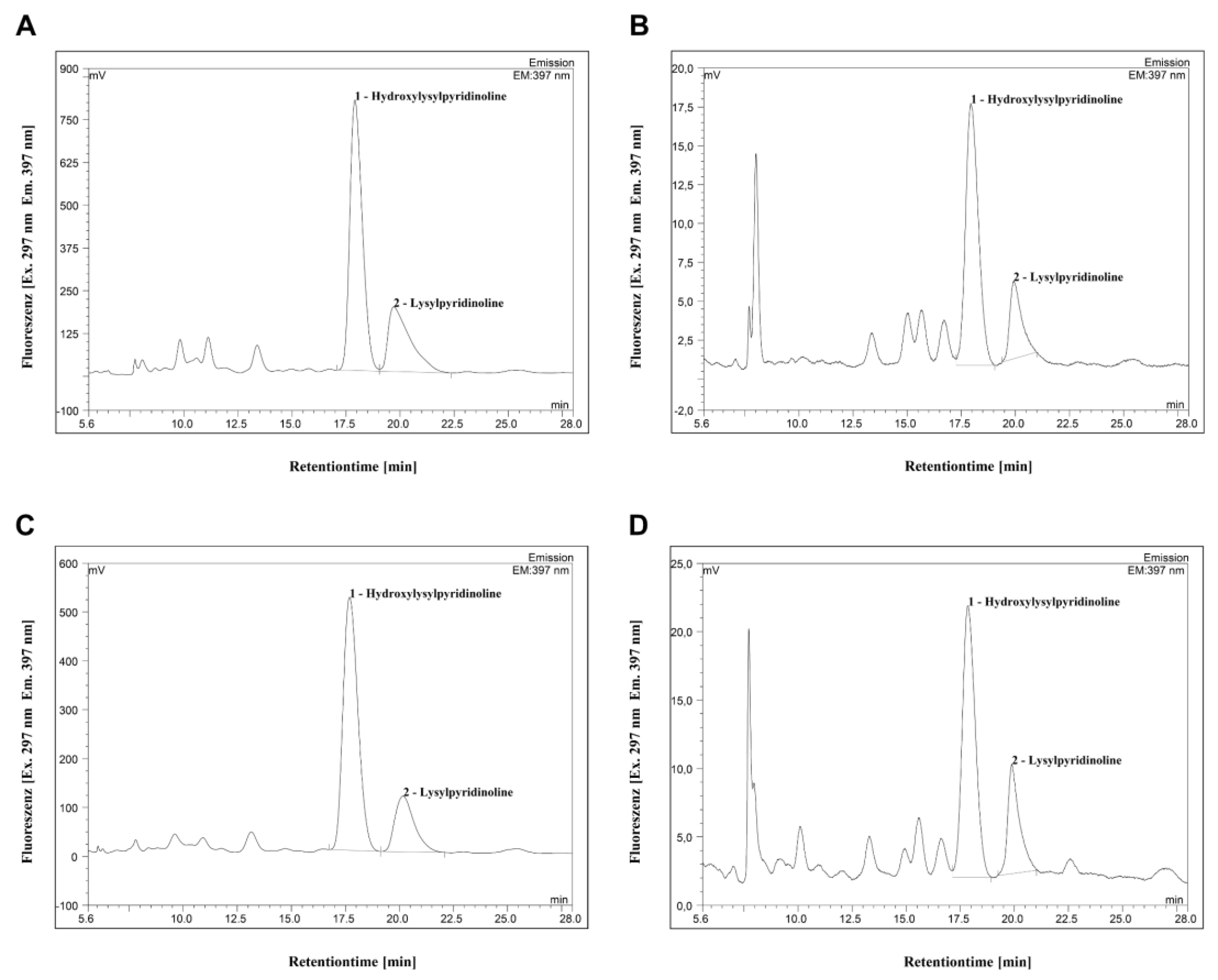
2.3. High-Performance Liquid Chromatographic Analysis of HP and LP
2.4. Quantification of Hydroxyproline
2.5. Detection of Type I and V Collagens Using Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
2.6. Statistics
3. Results
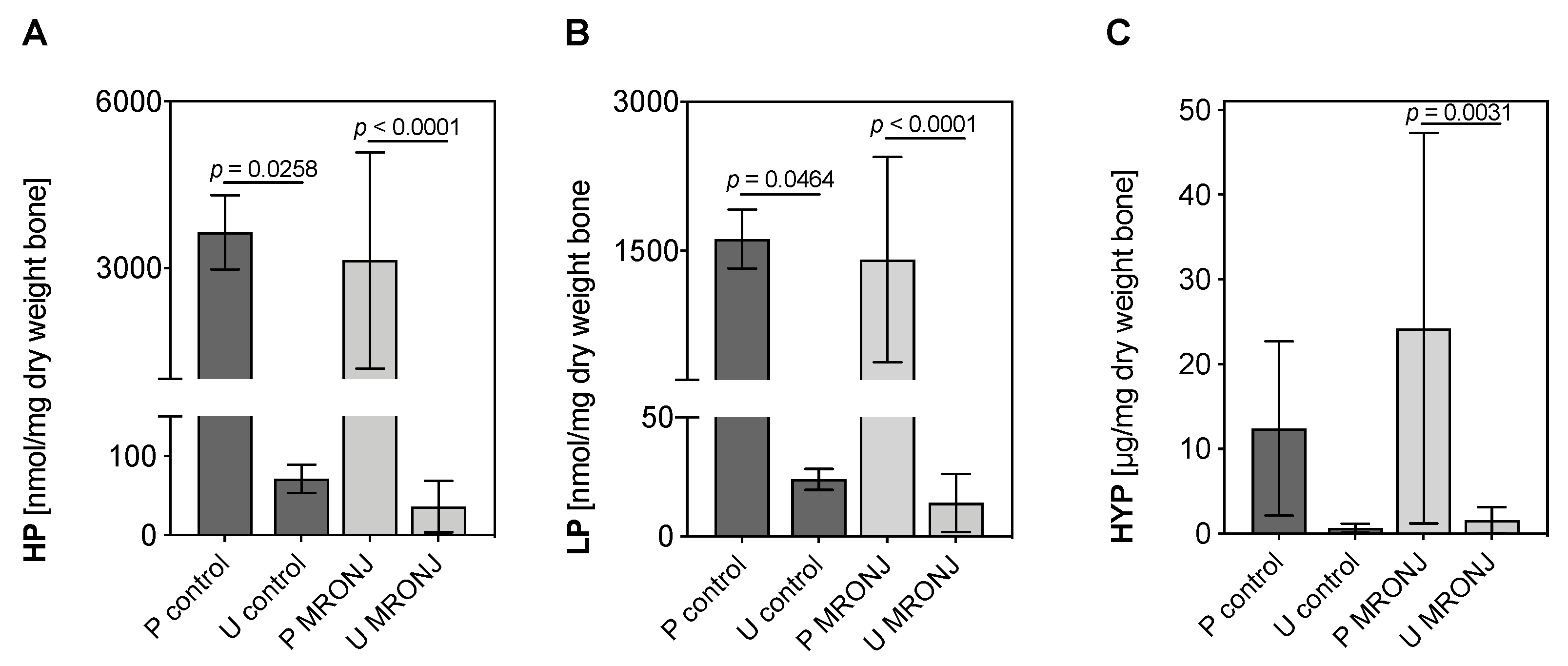
3.1. Quantification of Collagen Cross-Links HP, LP and Hyp
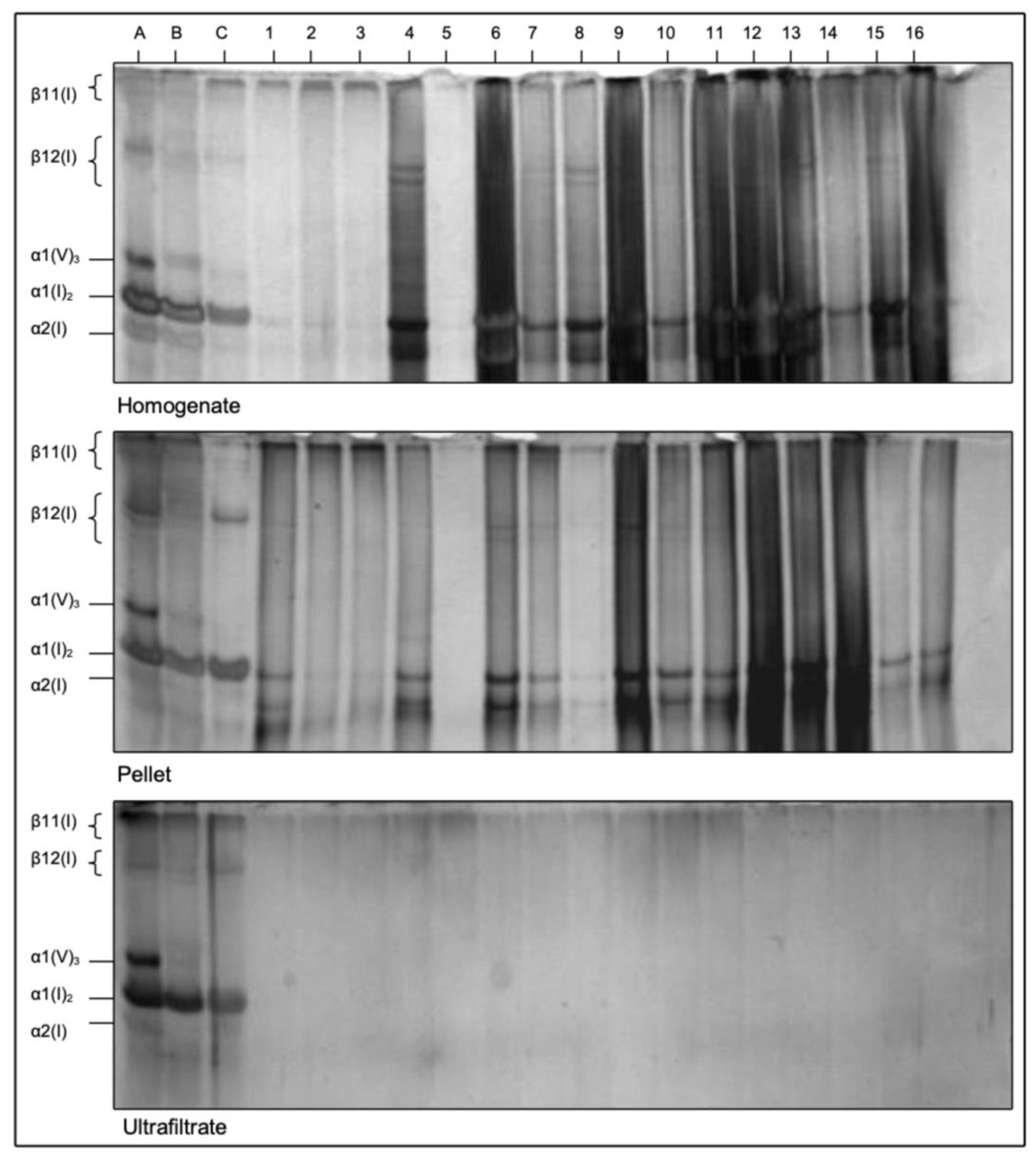
3.2. SDS-PAGE of Type I and V Collagen
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Boquete-Castro, A.; Gómez-Moreno, G.; Calvo-Guirado, J.L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin. Oral Implants Res. 2016, 27, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Antonuzzo, L.; Lunghi, A.; Petreni, P.; Brugia, M.; Laffi, A.; Giommoni, E.; Mela, M.M.; Mazzoni, F.; Balestri, V.; Costanzo, F.D. Osteonecrosis of the Jaw and Angiogenesis inhibitors: A Revival of a Rare but Serous Side Effect. Curr. Med. Chem. 2017, 24, 3068–3076. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef]
- Body, J.J.; Mancini, I. Bisphosphonates for cancer patients: Why, how, and when? Support. Care Cancer 2002, 10, 399–407. [Google Scholar] [CrossRef]
- Stopeck, A.T.; Lipton, A.; Body, J.J.; Steger, G.G.; Tonkin, K.; de Boer, R.H.; Lichinitser, M.; Fujiwara, Y.; Yardley, D.A.; Viniegra, M.; et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: A randomized, double-blind study. J. Clin. Oncol. 2010, 28, 5132–5139. [Google Scholar] [CrossRef]
- O’Carrigan, B.; Wong, M.H.; Willson, M.L.; Stockler, M.R.; Pavlakis, N.; Goodwin, A. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst. Rev. 2017, 10, Cd003474. [Google Scholar] [CrossRef]
- Dunford, J.E.; Thompson, K.; Coxon, F.P.; Luckman, S.P.; Hahn, F.M.; Poulter, C.D.; Ebetino, F.H.; Rogers, M.J. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001, 296, 235–242. [Google Scholar]
- Hanley, D.A.; Adachi, J.D.; Bell, A.; Brown, V. Denosumab: Mechanism of action and clinical outcomes. Int. J. Clin. Pract. 2012, 66, 1139–1146. [Google Scholar] [CrossRef]
- Boyce, B.F.; Li, J.; Xing, L.; Yao, Z. Bone Remodeling and the Role of TRAF3 in Osteoclastic Bone Resorption. Front. Immunol. 2018, 9, 2263. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.R.; Andersen, T.L.; Chavassieux, P.; Roux, J.P.; Delaisse, J.M. Bisphosphonates impair the onset of bone formation at remodeling sites. Bone 2021, 145, 115850. [Google Scholar] [CrossRef] [PubMed]
- Kos, M. Association of dental and periodontal status with bisphosphonate-related osteonecrosis of the jaws. A retrospective case controlled study. Arch. Med. Sci. 2014, 10, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Şahin, O.; Tatar, B.; Ekmekcioğlu, C.; Aliyev, T.; Odabaşı, O. Prevention of medication related osteonecrosis of the jaw after dentoalveolar surgery: An institution’s experience. J. Clin. Exp. Dent. 2020, 12, e771–e776. [Google Scholar] [CrossRef]
- Shuster, A.; Reiser, V.; Trejo, L.; Ianculovici, C.; Kleinman, S.; Kaplan, I. Comparison of the histopathological characteristics of osteomyelitis, medication-related osteonecrosis of the jaw, and osteoradionecrosis. Int. J. Oral Maxillofac. Surg. 2019, 48, 17–22. [Google Scholar] [CrossRef]
- Fondi, C.; Franchi, A. Definition of bone necrosis by the pathologist. Clin. Cases Miner. Bone Metab. 2007, 4, 21–26. [Google Scholar]
- Giudice, A.; Antonelli, A.; Muraca, D.; Fortunato, L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020, 31, 813–818. [Google Scholar]
- Açil, Y.; Springer, I.N.; Niehoff, P.; Gassling, V.; Warnke, P.H.; Açmaz, S.; Sönmez, T.T.; Kimmig, B.; Lefteris, V.; Wiltfang, J. Proof of direct radiogenic destruction of collagen in vitro. Strahlenther. Onkol. 2007, 183, 374–379. [Google Scholar] [CrossRef]
- Acil, Y.; Brinckmann, J.; Behrens, P.; Muller, P.K.; Batge, B. Semipreparative isolation of collagen types I, II, III and V by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroelution. J. Chromatogr. A 1997, 758, 313–318. [Google Scholar] [CrossRef]
- Açil, Y.; Müller, P.K. Rapid method for the isolation of the mature collagen cross-links, hydroxylysylpyridinoline and lysylpyridinoline. J. Chromatogr. A 1994, 664, 183–188. [Google Scholar] [CrossRef]
- Açil, Y.; Gierloff, M.; Behrens, C.; Möller, B.; Gassling, V.; Niehoff, P.; Wiltfang, J.; Simon, M. Effects of zoledronate on irradiated bone in vivo: Analysis of the collagen types I, V and their cross-links lysylpyridinoline, hydroxylysylpyridinoline and hydroxyproline. Calcif. Tissue Int. 2013, 92, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Springer, I.N.; Terheyden, H.; Dunsche, A.; Czech, N.; Suhr, M.A.; Tiemann, M.; Hedderich, J.; Açil, Y. Collagen crosslink excretion and staging of oral cancer. Br. J. Cancer 2003, 88, 1105–1110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed]
- Nyman, J.S.; Roy, A.; Acuna, R.L.; Gayle, H.J.; Reyes, M.J.; Tyler, J.H.; Dean, D.D.; Wang, X. Age-related effect on the concentration of collagen crosslinks in human osteonal and interstitial bone tissue. Bone 2006, 39, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Mücke, T.; Krestan, C.R.; Mitchell, D.A.; Kirschke, J.S.; Wutzl, A. Bisphosphonate and Medication-Related Osteonecrosis of the Jaw: A Review. Semin. Musculoskelet. Radiol. 2016, 20, 305–314. [Google Scholar] [PubMed]
- Akashi, M.; Wanifuchi, S.; Iwata, E.; Takeda, D.; Kusumoto, J.; Furudoi, S.; Komori, T. Differences between osteoradionecrosis and medication-related osteonecrosis of the jaw. Oral Maxillofac. Surg. 2018, 22, 59–63. [Google Scholar] [CrossRef]
- Li, J.; Schwimmbeck, P.L.; Tschope, C.; Leschka, S.; Husmann, L.; Rutschow, S.; Reichenbach, F.; Noutsias, M.; Kobalz, U.; Poller, W.; et al. Collagen degradation in a murine myocarditis model: Relevance of matrix metalloproteinase in association with inflammatory induction. Cardiovasc. Res. 2002, 56, 235–247. [Google Scholar] [CrossRef]
- Ben-David, D.; Livne, E.; Reznick, A.Z. The involvement of oxidants and NF-κB in cytokine-induced MMP-9 synthesis by bone marrow-derived osteoprogenitor cells. Inflamm. Res. 2012, 61, 673–688. [Google Scholar] [CrossRef]
- Thumbigere-Math, V.; Michalowicz, B.S.; de Jong, E.P.; Griffin, T.J.; Basi, D.L.; Hughes, P.J.; Tsai, M.L.; Swenson, K.K.; Rockwell, L.; Gopalakrishnan, R. Salivary proteomics in bisphosphonate-related osteonecrosis of the jaw. Oral Dis. 2015, 21, 46–56. [Google Scholar] [CrossRef]
- Nifosì, G.; Nifosì, L.; Nifosì, A.F. Mesenchymal stem cells in the treatment of osteonecrosis of the jaw. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 65–75. [Google Scholar] [CrossRef]
- Shi, Q.; Huo, N.; Wang, X.; Yang, S.; Wang, J.; Zhang, T. Exosomes from oral tissue stem cells: Biological effects and applications. Cell Biosci. 2020, 10, 108. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, A.; Chiarella, E.; Baudi, F.; Scardamaglia, P.; Antonelli, A.; Giudice, D.; Barni, T.; Fortunato, L.; Giudice, A. Dose-Dependent Effects of Zoledronic Acid on Human Periodontal Ligament Stem Cells: An In Vitro Pilot Study. Cell Transplant. 2020, 29, 963689720948497. [Google Scholar] [CrossRef] [PubMed]
- Delmas, P.D.; Eastell, R.; Garnero, P.; Seibel, M.J.; Stepan, J. The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos. Int. 2000, 11 (Suppl. 6), S2–S17. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Ferreras, M.; Karsdal, M.A.; Nicamhlaoibh, R.; Risteli, J.; Borel, O.; Qvist, P.; Delmas, P.D.; Foged, N.T.; Delaissé, J.M. The type I collagen fragments ICTP and CTX reveal distinct enzymatic pathways of bone collagen degradation. J. Bone Miner. Res. 2003, 18, 859–867. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Ohe, J.Y.; Kim, D.Y.; Chung, D.J.; Park, Y.D. Retrospective study of two biochemical markers for the risk assessment of oral bisphosphonate-related osteonecrosis of the jaws: Can they be utilized as risk markers? Clin. Oral Implants Res. 2011, 22, 100–105. [Google Scholar] [CrossRef]
- Fleisher, K.E.; Welch, G.; Kottal, S.; Craig, R.G.; Saxena, D.; Glickman, R.S. Predicting risk for bisphosphonate-related osteonecrosis of the jaws: CTX versus radiographic markers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 509–516. [Google Scholar] [CrossRef]
| ID | Birth Year/Sex | Diagnosis | Donor Site | Bisphosphonate | |
|---|---|---|---|---|---|
| 1 | 1940 | ♀ | Multiple Myeloma | Mandible | Zometa® + Aredia® |
| 2 | 1966 | ♂ | Prostate cancer | Mandible | Zometa® |
| 3 | 1943 | ♀ | Breast cancer | Maxilla | Zometa® + Aredia® |
| 4 | 1949 | ♂ | Multiple Myeloma | Mandible | Zometa® + Pamidronat |
| 5 | 1949 | ♂ | Lymphoma | Mandible | Zometa® + Ibandronat |
| 6 | 1945 | ♀ | Breast cancer | Mandible | Zometa® |
| 7 | 1949 | ♂ | Multiple Myeloma | Mandible | Zometa® + Aredia® |
| 8 | 1959 | ♀ | Multiple Myeloma | Mandible | Zometa® |
| 9 | 1934 | ♂ | Multiple Myeloma | Mandible | Zometa® + Ibandronat |
| 10 | 1938 | ♀ | Breast cancer | Mandible | Aredia® |
| 11 | 1923 | ♀ | Osteoporosis | Mandible | Zometa® + Pamidronat |
| 12 | 1949 | ♀ | Multiple Myeloma | Mandible | Zometa® + Ibandronat |
| 13 | 1933 | ♂ | Multiple Myeloma | Mandible | Zometa® + Pamidronat |
| 14 | 1939 | ♂ | Multiple Myeloma | Mandible | Zometa® + Pamidronat |
| 15 | 1937 | ♀ | Osteoporosis | Mandible | Zometa® + Pamidronat |
| Control Group (n = 3) | |||||
| 1947 | ♂ | Mandible | No antiresorptive medication | ||
| 1945 | ♀ | Mandible | No antiresorptive medication | ||
| 1941 | ♀ | Mandible | No antiresorptive medication | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acil, Y.; Weitkamp, J.-T.; Wieker, H.; Flörke, C.; Wiltfang, J.; Gülses, A. Organic Bone Matrix Component Type I and V Collagen Are Not Destructed in Bisphosphonate-Associated Osteonecrosis of the Jaws. Medicina 2022, 58, 1690. https://doi.org/10.3390/medicina58111690
Acil Y, Weitkamp J-T, Wieker H, Flörke C, Wiltfang J, Gülses A. Organic Bone Matrix Component Type I and V Collagen Are Not Destructed in Bisphosphonate-Associated Osteonecrosis of the Jaws. Medicina. 2022; 58(11):1690. https://doi.org/10.3390/medicina58111690
Chicago/Turabian StyleAcil, Yahya, Jan-Tobias Weitkamp, Henning Wieker, Christian Flörke, Jörg Wiltfang, and Aydin Gülses. 2022. "Organic Bone Matrix Component Type I and V Collagen Are Not Destructed in Bisphosphonate-Associated Osteonecrosis of the Jaws" Medicina 58, no. 11: 1690. https://doi.org/10.3390/medicina58111690
APA StyleAcil, Y., Weitkamp, J.-T., Wieker, H., Flörke, C., Wiltfang, J., & Gülses, A. (2022). Organic Bone Matrix Component Type I and V Collagen Are Not Destructed in Bisphosphonate-Associated Osteonecrosis of the Jaws. Medicina, 58(11), 1690. https://doi.org/10.3390/medicina58111690





