Spectacular and Prompt Response to Extracorporeal Photopheresis for Refractory Cutaneous Chronic Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation: A Case Report
Abstract
1. Introduction
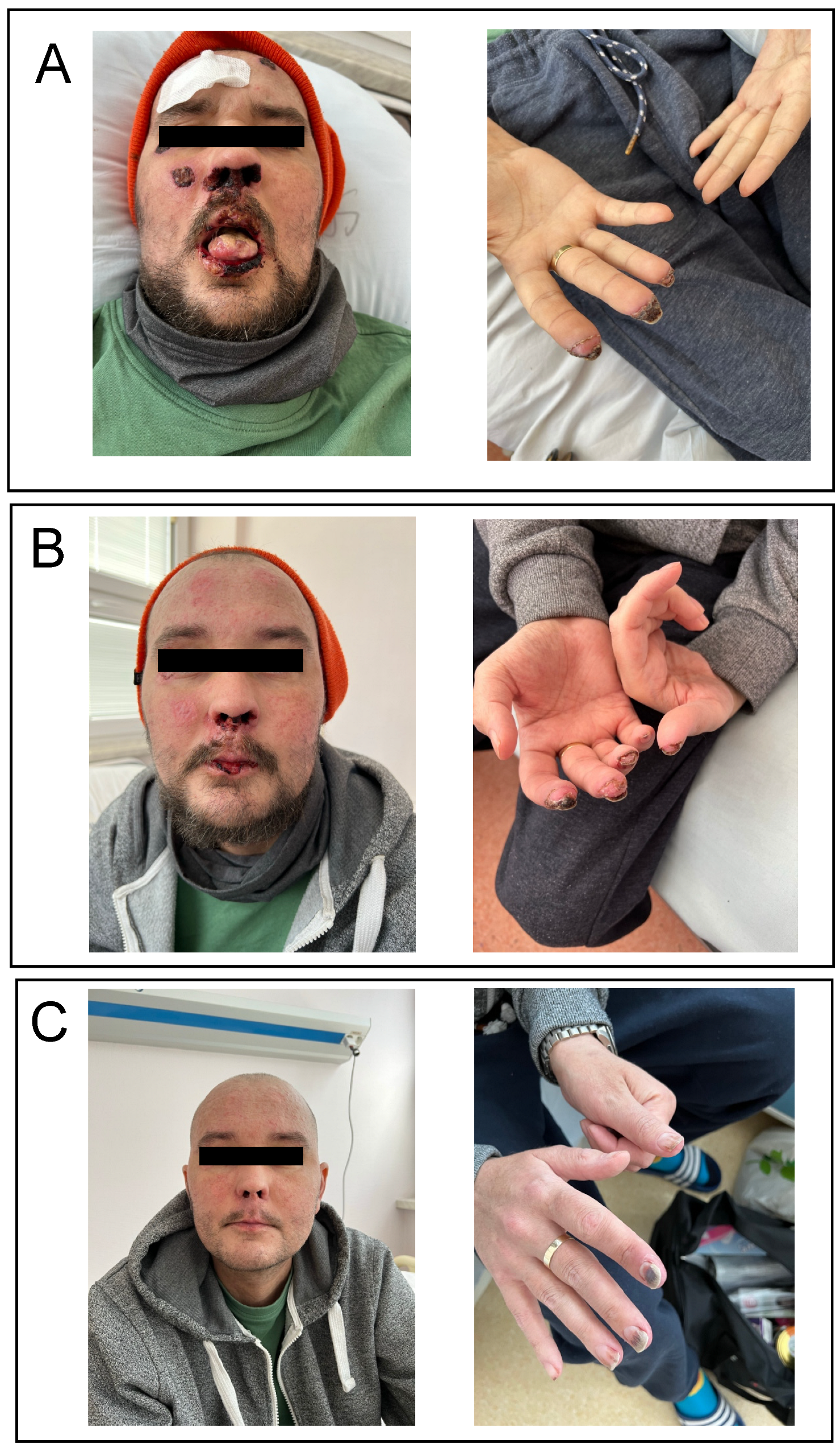
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohty, B.; Mohty, M. Long-term complications and side effects after allogenic hematopoietic stem cell transplantation: An update. Blood Cancer J. 2011, 1, e16. [Google Scholar] [CrossRef] [PubMed]
- Flowers, M.E.; Martin, P.J. How we treat chronic graft-versus-host disease. Blood 2015, 125, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Strong Rodrigues, K.; Oliveira-Ribeiro, C.; de Abreu Fiuza Gomes, S.; Knobler, R. Cutaneous graft-versus-host disease: Diagnosis and treatment. Am. J. Clin. Dermatol. 2018, 19, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Penack, O.; Marchetti, M.; Ruutu, T.; Aljurf, M.; Bacigalupo, A.; Bonifazi, F.; Ciceri, F.; Cornelissen, J.; Malladi, R.; Duarte, R.F.; et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: Updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020, 7, e157–e167. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Jantschitsch, C.; Knobler, R. Extracorporeal photopheresis-an overview. Front. Med. 2018, 5, 236. [Google Scholar] [CrossRef] [PubMed]
- Drexler, B.; Buser, A.; Infanti, L.; Stehle, G.; Halter, J.; Holbro, A. Extracorporeal photopheresis in graft-versus-host-disease. Transfus. Med. Hemother. 2020, 47, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Young, R.; Devine, S.; Hogan, W.J.; Ayuk, F.; Bunworasate, U.; Chanswangphuwana, C.; Efebera, Y.A.; Holler, E.; Litzow, M.; et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: A report from the Mount Sinai Acute GVHD International Consortium. Biol. Blood Marrow Transplant. 2016, 22, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Jagasia, M.H.; Greinix, H.T.; Flowers, M.E. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol. Blood Marrow Transplant. 2015, 21, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Maas-Bauer, K.; Kiote-Schmidt, C.; Bertz, H.; Apostolova, P.; Wäsch, R.; Ihorst, G.; Finke, J.; Zeiser, R. Ruxolitinib-ECP combination treatment for refractory severe chronic graft-versus-host disease. Bone Marrow Transplant. 2021, 56, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dalle, I.; Reljic, T.; Nishihori, T.; Antar, A.; Bazarbachi, A.; Djulbegovic, B.; Kumar, A.; Kharfan-Dabaja, M.A. Extracorporeal photopheresis in steroid-refractory acute or chronic graft-versus-host disease: Results of a systemic review of prospective studies. Biol. Blood Marrow Transplant. 2014, 20, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Oarbeascoa, G.; Lozano, M.L.; Guerra, M.L.; Amunarriz, C.; Saavedra, C.A.; Garcia-Gala, J.M.; Viejo, A.; Revilla, N.; Acosta Fleitas, C.; Arroyo, J.L.; et al. Grupo Español de Aféresis—Spanish Apheresis Group. Retrospective multicenter study of extracorporeal photopheresis in steroid-refractory acute and chronic graft-versus-host disease. Biol. Blood Marrow Transplant. 2020, 26, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Malek Mohammadi, A.; Norooznezhad, A.H.; Heshmati, F.; Alimoghaddam, K.; Ghavamzadeh, A. Extra corporeal photochemotherapy in steroid refractory graft versus host disease: A review of guidelines and recommendations. Transfus. Apher. Sci. 2017, 56, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Couriel, D.R.; Hosing, C.; Saliba, R.; Shpall, E.J.; Anderlini, P.; Rhodes, B.; Smith, V.; Khouri, I.; Giralt, S.; de Lima, M.; et al. Extracorporeal photochemotherapy for the treatment of steroid-resistant chronic GVHD. Blood 2006, 107, 3074–3080. [Google Scholar] [CrossRef] [PubMed]
- Changsirikulchai, S.; Myerson, D.; Guthrie, K.A.; McDonald, G.B.; Alpers, C.E.; Hingorani, S.R. Renal thrombotic microangiopathy after hematopoietic cell transplant: Role of GVHD in pathogenesis. Clin. J. Am. Soc. Nephrol. 2009, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Jodele, S.; Laskin, B.L.; Dandoy, C.E.; Myers, K.C.; El-Bietar, J.; Davies, S.M.; Goebel, J.; Dixon, B.P. A new paradigm: Diagnosis and management of HSCT-associated thrombotic microangiopathy as multi-system endothelial injury. Blood Rev. 2015, 29, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Lämmle, B. Hematopoietic stem cell transplantation-associated thrombotic microangiopathy: Pathophysiology and differentiation from graft versus host disease. Thromb. Haemost. 2019, 119, 1382. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Chrysanthopoulou, A.; Sakellari, I.; Batsis, I.; Mallouri, D.; Touloumenidou, T.; Papalexandri, A.; Mitsios, A.; Arampatzioglou, A.; Ritis, K.; et al. Linking complement activation, coagulation and neutrophils in transplant-associated thrombotic microangiopathy. Thromb. Haemost 2019, 119, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, J. Hematopoietic cell transplantation-associated thrombotic microangiopathy: A review of pathophysiology, diagnosis and treatment. J. Blood Med. 2016, 7, 181–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aubin, F.; Mousson, C. Ultraviolet light-induced regulatory (suppressor) T-cells: An approach for promoting induction of operational allograft tolerance? Transplantation 2004, 77, S29–S31. [Google Scholar] [CrossRef] [PubMed]
- Whittle, R.; Taylor, P.C. Circulating B-cell activating factor level predicts clinical response of chronic graft-versus-host-disease to extracorporeal photopheresis. Blood 2011, 118, 6446–6449. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spałek, A.; Grygoruk-Wiśniowska, I.; Gruenpeter, K.; Panz-Klapuch, M.; Helbig, G. Spectacular and Prompt Response to Extracorporeal Photopheresis for Refractory Cutaneous Chronic Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation: A Case Report. Medicina 2022, 58, 1722. https://doi.org/10.3390/medicina58121722
Spałek A, Grygoruk-Wiśniowska I, Gruenpeter K, Panz-Klapuch M, Helbig G. Spectacular and Prompt Response to Extracorporeal Photopheresis for Refractory Cutaneous Chronic Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation: A Case Report. Medicina. 2022; 58(12):1722. https://doi.org/10.3390/medicina58121722
Chicago/Turabian StyleSpałek, Adrianna, Iwona Grygoruk-Wiśniowska, Karolina Gruenpeter, Marta Panz-Klapuch, and Grzegorz Helbig. 2022. "Spectacular and Prompt Response to Extracorporeal Photopheresis for Refractory Cutaneous Chronic Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation: A Case Report" Medicina 58, no. 12: 1722. https://doi.org/10.3390/medicina58121722
APA StyleSpałek, A., Grygoruk-Wiśniowska, I., Gruenpeter, K., Panz-Klapuch, M., & Helbig, G. (2022). Spectacular and Prompt Response to Extracorporeal Photopheresis for Refractory Cutaneous Chronic Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation: A Case Report. Medicina, 58(12), 1722. https://doi.org/10.3390/medicina58121722





