Topographic and Morphometric Study of the Foramen Spinosum of the Skull and Its Clinical Correlation
Abstract
1. Introduction
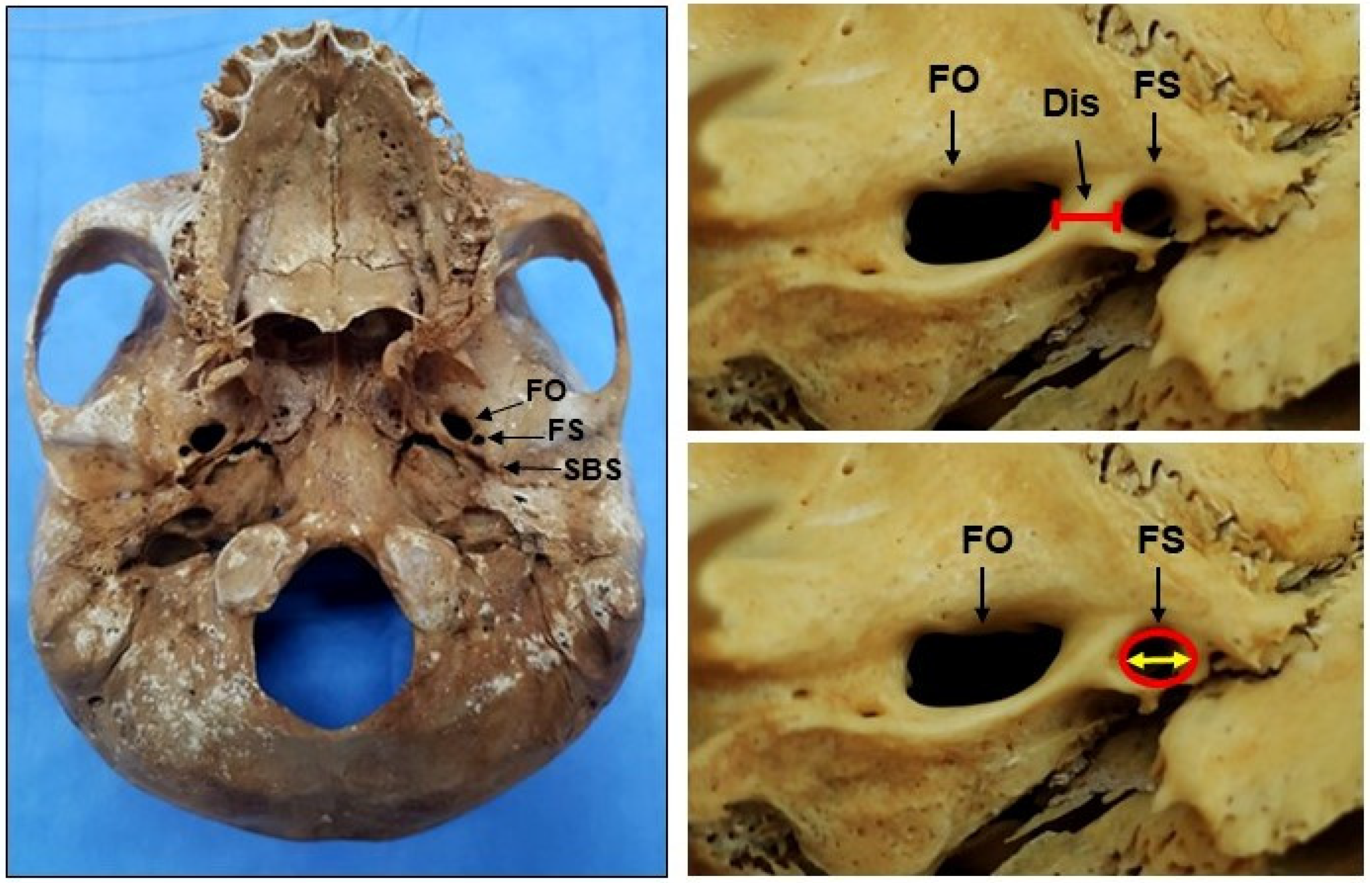
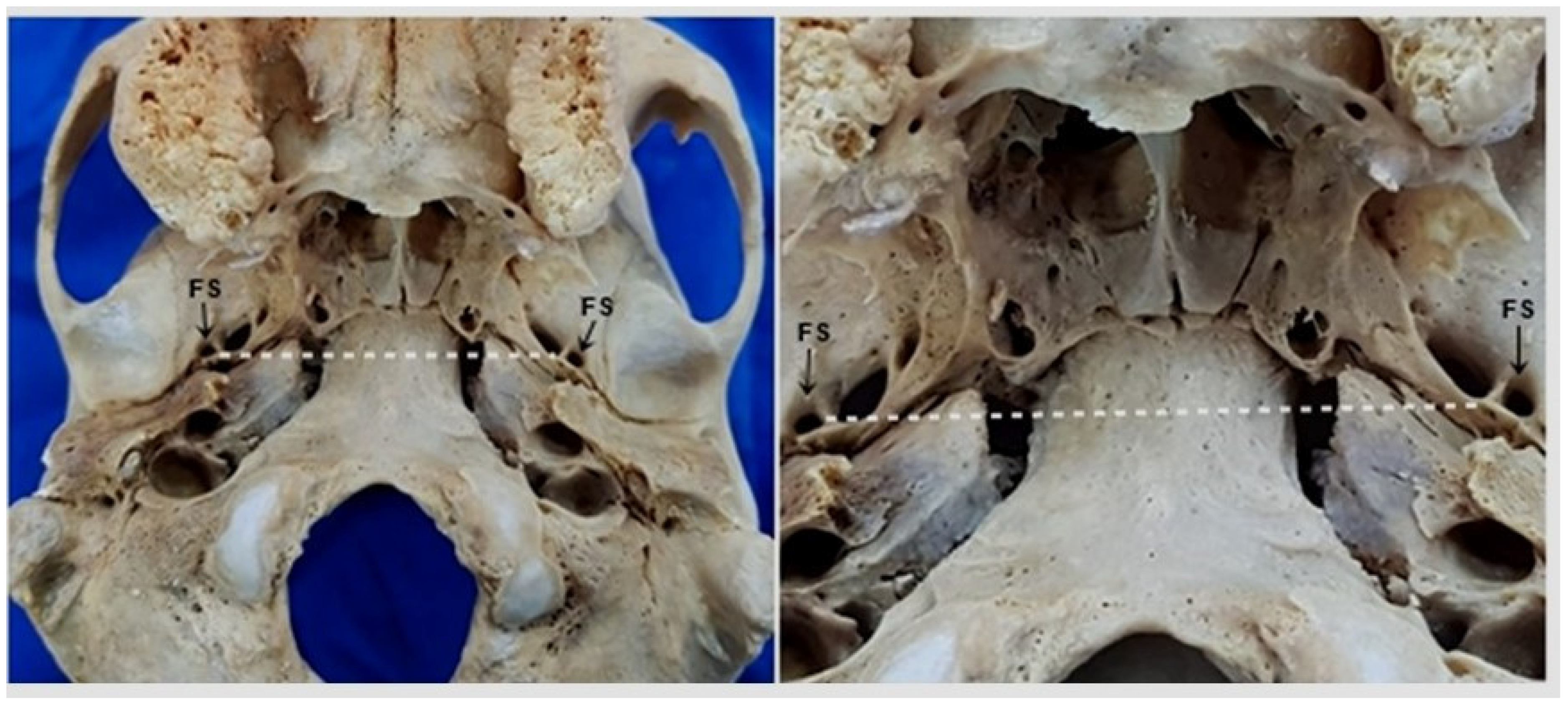
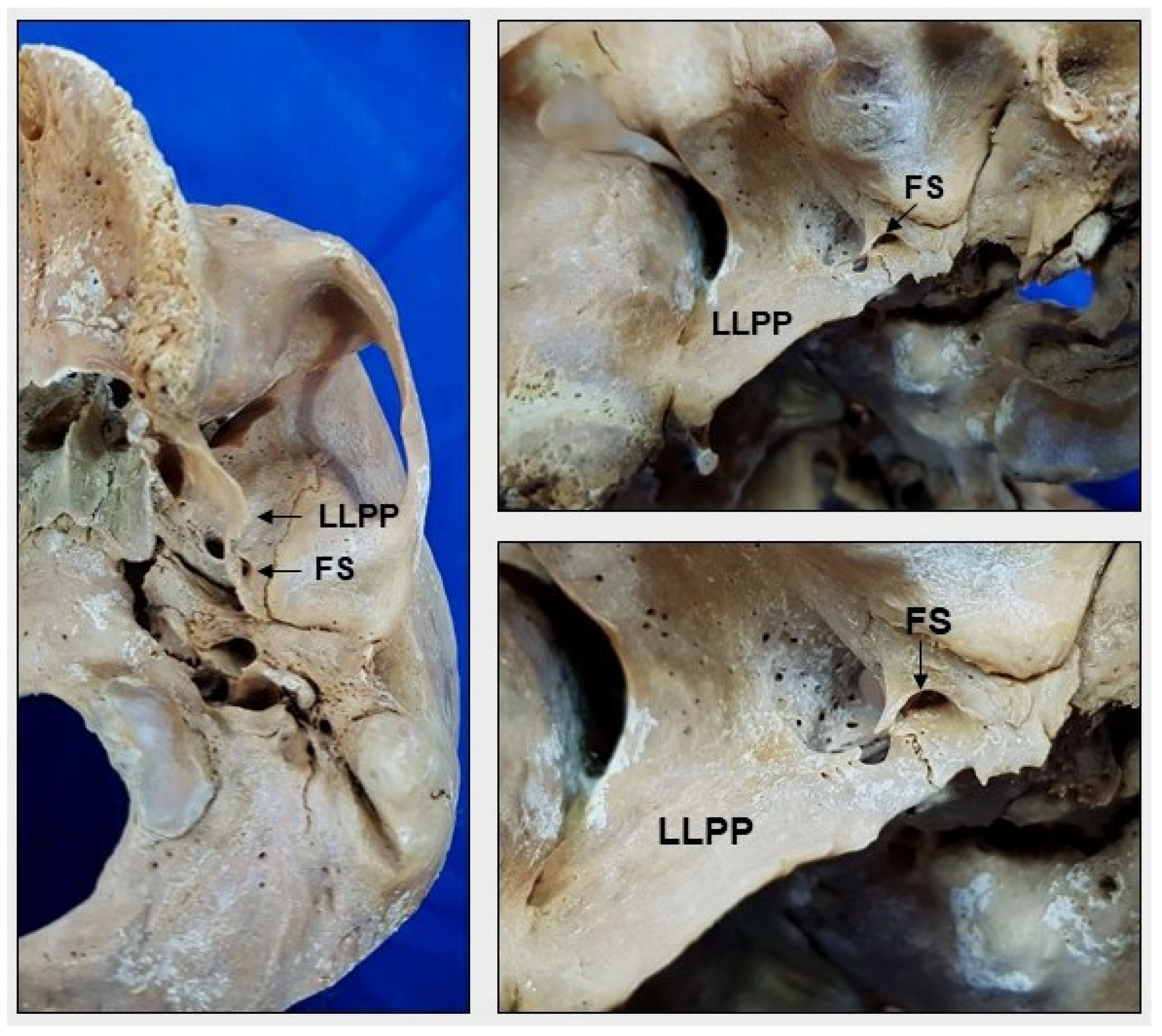
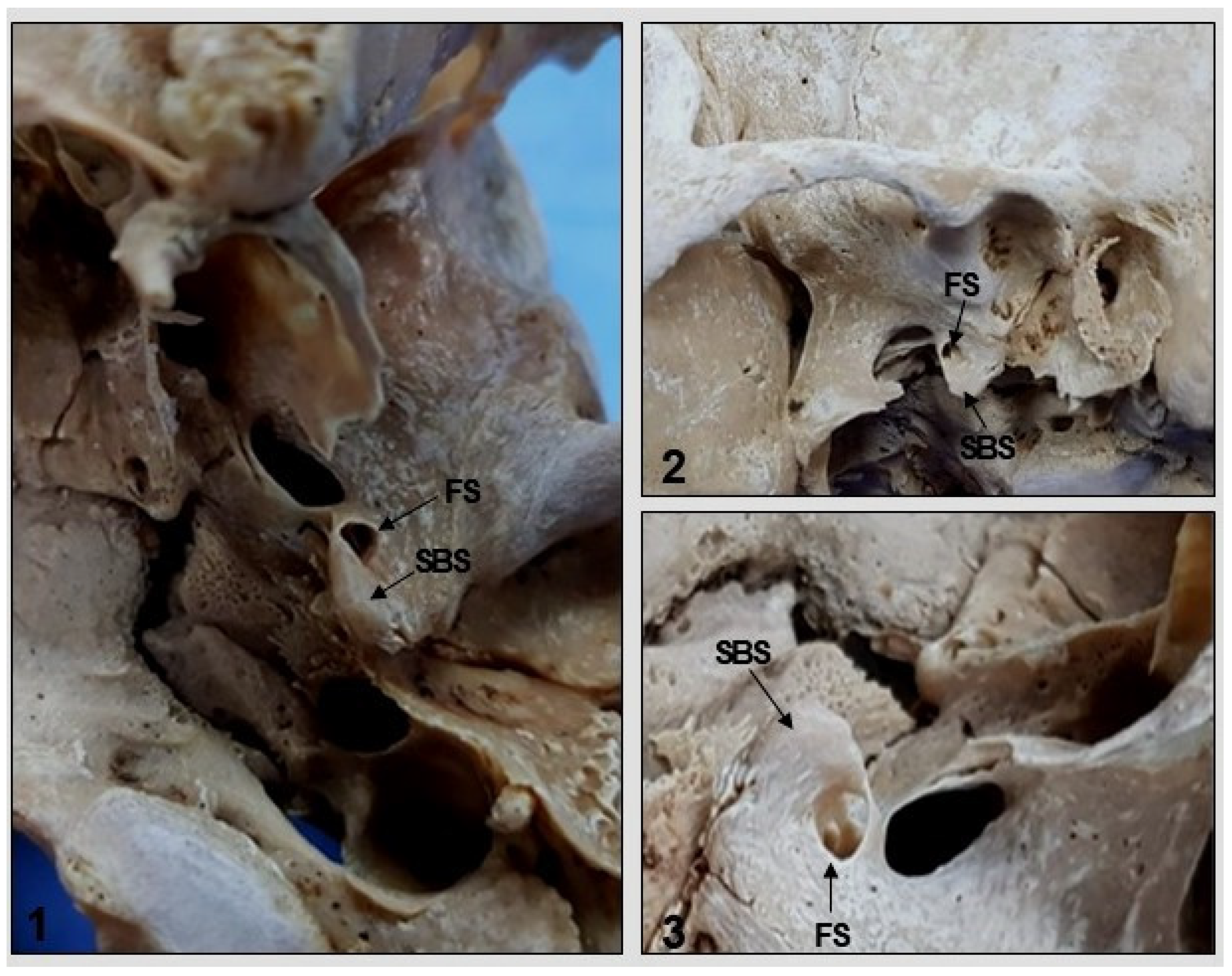
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krayenbühl, N.; Isolan, G.R.; Al-Mefty, O. The foramen spinosum: A landmark in middle fossa surgery. Neurosurg. Rev. 2008, 31, 397–401. [Google Scholar] [CrossRef]
- Radosevic, D.; Maric, D.; Ivanovic, D. Human skull base asymmetry analysis. Int. J. Morphol. 2020, 38, 1566–1570. [Google Scholar] [CrossRef]
- Medeiros, R.J. Anatomical Description of Transmaxillary Access Based on Skull: Study on Human Cadaver. Graduation Monograph, Federal University of Rio Grande do Norte, Natal. 2019. Available online: https://repositorio.ufrn.br/handle/123456789/39065 (accessed on 23 September 2022).
- Novaes, A.R. Factors Related to Failure of Inferior Alveolar Nerve Block. Graduation Monograph; University of Brasilia: Brasilia, Brazil, 2018. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy; The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone: London, UK, 2004; pp. 462–467. [Google Scholar]
- Moore, K.L.; Dalley, A.F.; Agur, A.M.R. Clinically Oriented Anatomy, 8th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2019; p. 1295. [Google Scholar]
- Campos, D.; Silveira, C.H.; Jotz, G.P.; Malysz, T. Bony tunnel formation associated with the distal segment of the frontal branch of the middle meningeal artery. J. Neurol. Surg. B Skull Base 2019, 80, 480–483. [Google Scholar] [CrossRef]
- Ellwanger, J.H.; Campos, D.d. Abnormality of the Foramen Spinosum due to a Variation in the Trajectory of the Middle Meningeal Artery: A Case Report in Human. J. Neurol Surg. Rep. 2013, 74, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Shotar, E.; Premat, K.; Lenck, S.; Degos, V.; Marijon, P.; Pouvelle, A.; Pouliquen, G.; Mouyal, S.; Abi Jaoude, S.; Sourour, N.A.; et al. Angiographic Anatomy of the Middle Meningeal Artery in Relation to Chronic Subdural Hematoma Embolization. Clin. Neuroradiol. 2022, 32, 57–67. [Google Scholar] [CrossRef]
- Natali, A.L.; Reddy, V.; Leo, J.T. Neuroanatomy, Middle Meningeal Arteries. In Stat Pearls; Stat Pearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kuruvilla, A.; Aguwa, A.N.; Lee, A.W.; Xavier, A.R. Anomalous origin of the middle meningeal artery from the posterior inferior cerebellar artery. J. Neuroimaging 2011, 21, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, K.L.; Zagami, A.S.; Goadsby, P.J. Stimulation of the middle meningeal artery leads to Fos expression in the trigeminocervical nucleus: A comparative study of monkey and cat. J. Anat. 1999, 194, 579–588. [Google Scholar] [CrossRef]
- Ustün, M.E.; Büyükmumcu, M.; Seker, M.; Karabulut, A.K.; Uysal, I.I.; Ziylan, T. Possibility of middle meningeal artery-to-petrous internal carotid artery bypass: An anatomic study. In Skull Base: An Interdisciplinary Approach, 14th ed.; Thieme Medical Publishers: New York, NY, USA, 2004; pp. 53–156. [Google Scholar] [CrossRef]
- Nikolova, S.Y.; Toneva, D.H.; Yordanov, Y.A.; Lazarov, N.E. Absence of foramen spinosum and abnormal middle meningeal artery in cranial series. J. Biol. Clin. Anthropol. 2012, 69, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Asari, M.A.; Hassan, A. Anatomic variants of foramen ovale and spinosum in human skulls. Int. J. Morphol. 2012, 30, 445–449. [Google Scholar] [CrossRef]
- Carvalho, B.V.; Gaiotti, J.O.; Diniz, R.L.F.C.; Ribeiro, M.A.; Motta, E.G.P.C.; Moreira, W. Persistence of stapedial artery: A case report. Radiol. Bras. 2013, 46, 184–186. [Google Scholar] [CrossRef]
- Lazarus, L.; Naidoo, N.; Satyapal, K.S. An osteometric evaluation of the foramen spinosum and venosum. Int. J. Morphol. 2015, 33, 452–458. [Google Scholar] [CrossRef][Green Version]
- Dilenge, D.; Ascherl, G.F., Jr. Variations of the ophthalmic and middle meningeal arteries: Relation to the embryonic stapedial artery. Am. J. Neuroradiol. 1980, 1, 45–54. [Google Scholar]
- Aguiar, G.; Silva, J.; Souza, R.; Acioly, M.A. Skull base fracture involving the foramen spinosum—An indirect sign of middle meningeal artery lesion: Case report and literature review. Turk. Neurosurg. 2015, 25, 317–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tacconi, V.F.S.; Barroso, L.K.V.; Azevedo, N.B.C.; Souza, T.R.; Matias, C.T.D. Epidural hematoma: A systematic review. J. Morphol. Sci. 2021, 38, 115–120. [Google Scholar] [CrossRef]
- Konuthula, N.; Perez, F.A.; Maga, A.M.; Abuzeid, W.; Moe, K.; Hannaford, B.; Bly, R.A. Automated atlas-based segmentation for skull base surgical planning. Int. J. Comput. Assist. Radiol. Surg. 2021, 16, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yi, Z. A New Bony Anatomical Landmark for Lateral Skull Base Surgery. J. Craniofacial Surg. 2020, 31, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Berge, J.K.; Bergman, R.A. Variations in size and in symmetry of foramina of the human skull. Clin. Anat. 2001, 14, 406–413. [Google Scholar] [CrossRef]
- Sophia, M.M.; Kalpana, R. A study on foramen spinosum. Int. J. Health Sci. Res. 2015, 5, 187–193. [Google Scholar]
- Khairnar, K.B.; Bhusar, P.A. An anatomical study on the foramen ovale and the foramen spinosum. J. Clin. Diagn. Res. 2013, 7, 427–429. [Google Scholar] [CrossRef]
- Edwards, B.; Wang, J.M.; Iwanaga, J.; Loukas, M.; Tubbs, R.S. Cranial nerve foramina. Part I: A review of the anatomy and pathology of cranial nerve foramina of the anterior and middle fossa. Cureus J. Med. Sci. 2018, 10, e2172. [Google Scholar] [CrossRef]
- Standring, S. Gray’s Anatomy; The Anatomical Basis of Clinical Practice, 40th ed.; Elsevier Churchill Livingstone: London, UK, 2008; pp. 424–425, 431–432. [Google Scholar]
- Varalakshmi, K.L.; Nayak, J.; Sangeeta, M. Anatomical Study of Foramen Ovale and Foramen Spinosum in the Norma Basalis with Clinical Correlation. Acta Sci. Orthop. 2021, 5, 21–26. [Google Scholar] [CrossRef]
- Abunimer, A.; Aiken, A.; Baugnon, K.; Wu, X. Central skull base anatomy and pathology: A review. Semin. Ultrasound CT MRI 2021, 42, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Shapey, J.; Thomas, N.W.M.; Connor, S.E.J. Neurosurgical Approaches to the Skull Base: A Guide for the Radiologist. Neuroimaging Clin. N. Am. 2021, 31, 409–431. [Google Scholar] [CrossRef]
- Braga, J.; Crubezy, E.; Elyaqtine, M. The posterior border of the sphenoid greater wing and its phylogenetic usefulness in human evolution. Am. J. Phys. Anthropol. 1998, 107, 387–399. [Google Scholar] [CrossRef]
- Worku, M.G.; Clarke, E. Morphometric analysis of the foramen spinosum and variations of its shape, number, and relation to the spine of the sphenoid bone. Transl. Res. Anat. 2021, 24, 100124. [Google Scholar] [CrossRef]
- Lindblom, K. A Roentgenographic Study of the Vascular Channels of the Skull. With Special Reference to Intracranial Tumors and Arteriovenous Aneurysms; Norstedt: Stockholm, Sweden, 1936; pp. 33–35. [Google Scholar]
- Royle, G.; Motson, R. An anomalous origin of the middle meningeal artery. J. Neurol. Neurosurg. Psychiatry 1973, 36, 874–876. [Google Scholar] [CrossRef][Green Version]
- Ginsberg, L.E.; Prett, S.W.; Chen, M.Y.; Elster, A.D. Skull-base foramina of the middle cranial fossa: Reassessment of normal variation with high-resolution CT. Am. J. Neuroradiol. 1994, 15, 283–291. [Google Scholar]
- Teul, I.; Czerwiński, F.; Gawlikowska, A.; Konstanty-Kurkiewicz, V.; Sławiński, G. Asymmetry of the ovale and spinous foramina in mediaeval and contemporary skulls in radiological examinations. Folia Morphol. 2002, 61, 147–152. [Google Scholar]
- Naqshi, B.F.; Shah, A.B.; Gupta, S. Variations in foramen ovale and foramen spinosum in human skulls of North Indian population. Int. J. Contemp. Med. Res. 2017, 4, 2262–2265. [Google Scholar]
- Tewari, S.; Gupta, C.; Palimar, V.; Kathur, S.G. Morphometric analysis of foramen spinosum in South Indian population. Acta Med. Iran. 2018, 56, 113–118. [Google Scholar]
- Chanda, C.; Singh, K.K.P.; Ranjan, R.; Prasad, R. Anatomical variations of foramen spinosum in adult human skulls of Jharkhand population. J. Evol. Med. Dent. Sci. 2019, 18, 1–7. [Google Scholar] [CrossRef]
- Lang, J.; Maier, R.; Schafhauser, O. Postnatal enlargement of the foramina rotundum, ovale et spinosum and their topographical changes. Anat. Anz. 1984, 156, 351–387. [Google Scholar]
- Yanagi, S. Developmental studies on the foramen rotundum, foramen ovale and foramen spinosum of the human sphenoid bone. Hokkaido J. Med. Sci. 1987, 62, 485–496. [Google Scholar] [PubMed]
- Somesh, M.S.; Murlimanju, B.V.; Krishnamurthy, A.; Sridevi, H.B. An anatomical study of foramen spinosum in South Indian dry skulls with its emphasis on morphology and morphometry. Int. J. Anat. Res. 2015, 3, 1034–1038. [Google Scholar] [CrossRef]
- Rai, A.L.; Gupta, N.; Rohatgi, R. Anatomical variations of foramen spinosum. Innov. J. Med. Health Sci. 2012, 2, 86–88. [Google Scholar]
- Reymond, J.; Charuta, A.; Wysocki, J. The morphology and morphometry of the foramina of the greater wing of the human sphenoid bone. Folia Morphol. 2005, 64, 188–193. [Google Scholar]
- Carolineberry, A.; Berry, R.J. Epigenetic variation in the human cranium. J. Anat. 1967, 101, 361–379. [Google Scholar]
- Wang, T.M.; Kuo, K.J.; Shih, C.; Ho, L.L.; Liu, J.C. Assessment of the relative locations of the greater palatine foramen in adult Chinese skulls. Acta Anatomica 1988, 132, 182–186. [Google Scholar] [CrossRef]
- Adachi, B. Das Arteriensystem der Japaner. Band 1; Verlag der Kaiserlich Japanischen Universität zu Kyoto: Kyoto, Japan, 1928; pp. 20–71. [Google Scholar]
- Kwathai, L.; Namonta, K.; Rungruang, T.; Chaisuksunt, V.; Apinhasmit, W.; Chompoopong, S. Anatomic and morphometric consideration for external landmarks of foramen spinosum in Thai dry skulls. Siriraj Med. J. 2012, 64, 26–29. [Google Scholar]
- Mandavi, S.; Mishra, A. Varying positions of foramen spinosum in relation to spine of sphenoid. J. Anat. Soc. India 2009, 58, 144–148. [Google Scholar]
- Osunwoke, E.A.; Mbadugha, C.C.; Orish, C.N.; Oghenemavwe, E.L.; Ukah, C.J. A morphometric study of foramen ovale and foramen spinosum of the human sphenoid bone in the southern Nigerian population. J. Appl. Biosci. 2010, 26, 1631–1635. [Google Scholar]
- Kulkarni, S.P.; Nikade, V.V. A morphometric study of foramen ovale and foramen spinosum in dried Indian human skulls. Int. J. Recent Trends Sci. Technol. 2013, 7, 74–75. [Google Scholar]
- Stevens, S.M.; Walters, Z.A.; Tawfik, K.; Samy, R.N. Two consecutive cases of persistent stapedial artery managed with a carbon dioxide laser. Ann. Otol. Rhinol. Laryngol. 2018, 127, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sattur, M.G.; Spiotta, A.M. Anomalous “middle” meningeal artery from basilar artery and implications for neuroendovascular surgery: Case report and review of literature. World Neurosurg. 2019, 133, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, K.Y. Anomalous origin of the middle meningeal arter—Case report with review of literature. Ann. Indian Acad. Neurol. 2001, 4, 83–87. [Google Scholar]
- Cvetko, E.; Bosnjak, R. Unilateral absence of foramen spinosum with bilateral ophthalmic origin of the middle meningeal artery: Case report and review of the literature. Folia Morphol. 2014, 73, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Buchaim, R.L.; Andreo, J.C.; Rodrigues, A.D.; Gonçalves, J.B.; Daré, L.R.; Rosa Júnior, G.M.; Buchaim, D.V.; Oliveira, J.A. Multidisciplinary approach in the teaching of dental sculpture and anatomy. Int. J. Morphol. 2014, 32, 399–403. [Google Scholar] [CrossRef]




| Female | Minimum | Maximum | Mean |
| Diameter | 1.68 mm | 3.23 mm | 2.45 mm |
| Length | 5.30 mm | 10.15 mm | 7.72 mm |
| FS–FO distance | 0.36 mm | 3.21 mm | 1.88 mm |
| FS–SM distance | 8.8 mm | 10.97 mm | 9.93 mm |
| FS–PT distance | 9.52 mm | 12.25 mm | 10.79 mm |
| Male | Minimum | Maximum | Mean |
| Diameter | 2.27 mm | 3.43 mm | 2.69 mm |
| Length | 7.14 mm | 10.79 mm | 8.48 mm |
| FS–FO distance | 0.5 mm | 4.32 mm | 2.87 mm |
| FS–SM distance | 8.66 mm | 10.78 mm | 9.99 mm |
| FS–PT distance | 9.35 mm | 11.59 mm | 10.78 mm |
| Female | Minimum | Maximum | Mean |
| Diameter | 2.10 mm | 4.70 mm | 2.89 mm |
| Length | 6.60 mm | 14.78 mm | 9.09 mm |
| FS–FO distance | 0.49 mm | 5.24 mm | 2.68 mm |
| FS–SM distance | 8.80 mm | 10.34 mm | 9.68 mm |
| FS–PT distance | 9.13 mm | 11.38 mm | 10.0 mm |
| Male | Minimum | Maximum | Mean |
| Diameter | 1.99 mm | 3.43 mm | 2.62 mm |
| Length | 6.26 mm | 10.77 mm | 8.26 mm |
| FS–FO distance | 0.79 mm | 5.76 mm | 2.97 mm |
| FS–SM distance | 8.16 mm | 10.78 mm | 9.53 mm |
| FS–PT distance | 8.37 mm | 11.76 mm | 10.06 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugano, G.T.; Pauris, C.C.; Silva, Y.B.e.; Pandini, F.E.; Palermo, R.B.S.; Buchaim, D.V.; Buchaim, R.L.; Chacon, E.L.; Aparecida de Castro, C.; Pagani, B.T.; et al. Topographic and Morphometric Study of the Foramen Spinosum of the Skull and Its Clinical Correlation. Medicina 2022, 58, 1740. https://doi.org/10.3390/medicina58121740
Sugano GT, Pauris CC, Silva YBe, Pandini FE, Palermo RBS, Buchaim DV, Buchaim RL, Chacon EL, Aparecida de Castro C, Pagani BT, et al. Topographic and Morphometric Study of the Foramen Spinosum of the Skull and Its Clinical Correlation. Medicina. 2022; 58(12):1740. https://doi.org/10.3390/medicina58121740
Chicago/Turabian StyleSugano, Gustavo Tenório, Carolina Chen Pauris, Yggor Biloria e Silva, Fabrício Egídio Pandini, Raíssa Balabem Said Palermo, Daniela Vieira Buchaim, Rogerio Leone Buchaim, Erivelto Luís Chacon, Cynthia Aparecida de Castro, Bruna Trazzi Pagani, and et al. 2022. "Topographic and Morphometric Study of the Foramen Spinosum of the Skull and Its Clinical Correlation" Medicina 58, no. 12: 1740. https://doi.org/10.3390/medicina58121740
APA StyleSugano, G. T., Pauris, C. C., Silva, Y. B. e., Pandini, F. E., Palermo, R. B. S., Buchaim, D. V., Buchaim, R. L., Chacon, E. L., Aparecida de Castro, C., Pagani, B. T., & Cunha, M. R. d. (2022). Topographic and Morphometric Study of the Foramen Spinosum of the Skull and Its Clinical Correlation. Medicina, 58(12), 1740. https://doi.org/10.3390/medicina58121740








