Fibrinogen-like Protein 1 as a Predictive Marker for the Incidence of Severe Acute Pancreatitis and Infectious Pancreatic Necrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
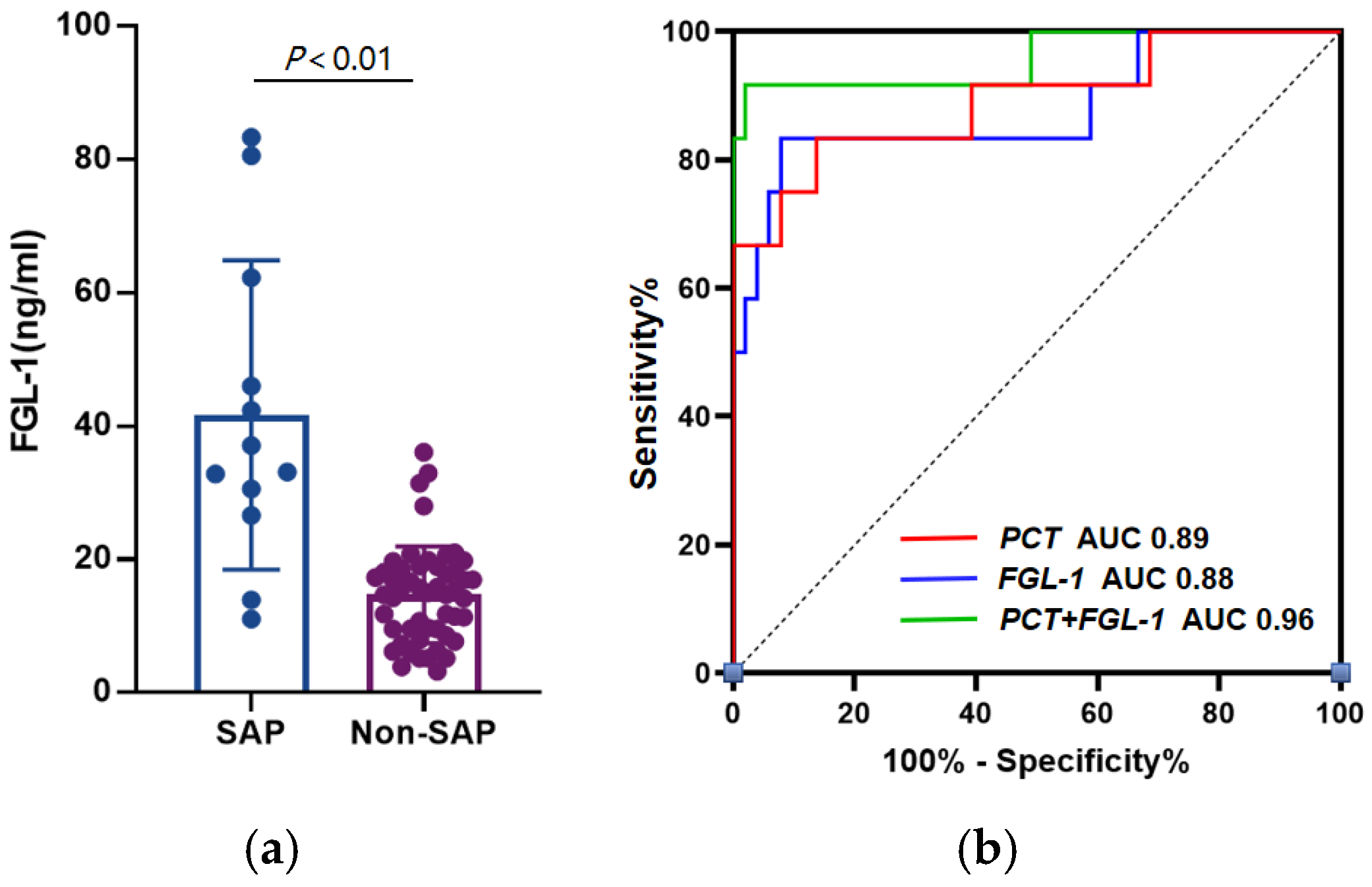
3.2. FGL-1 and PCT Shows Excellent Diagnostic Power in SAP
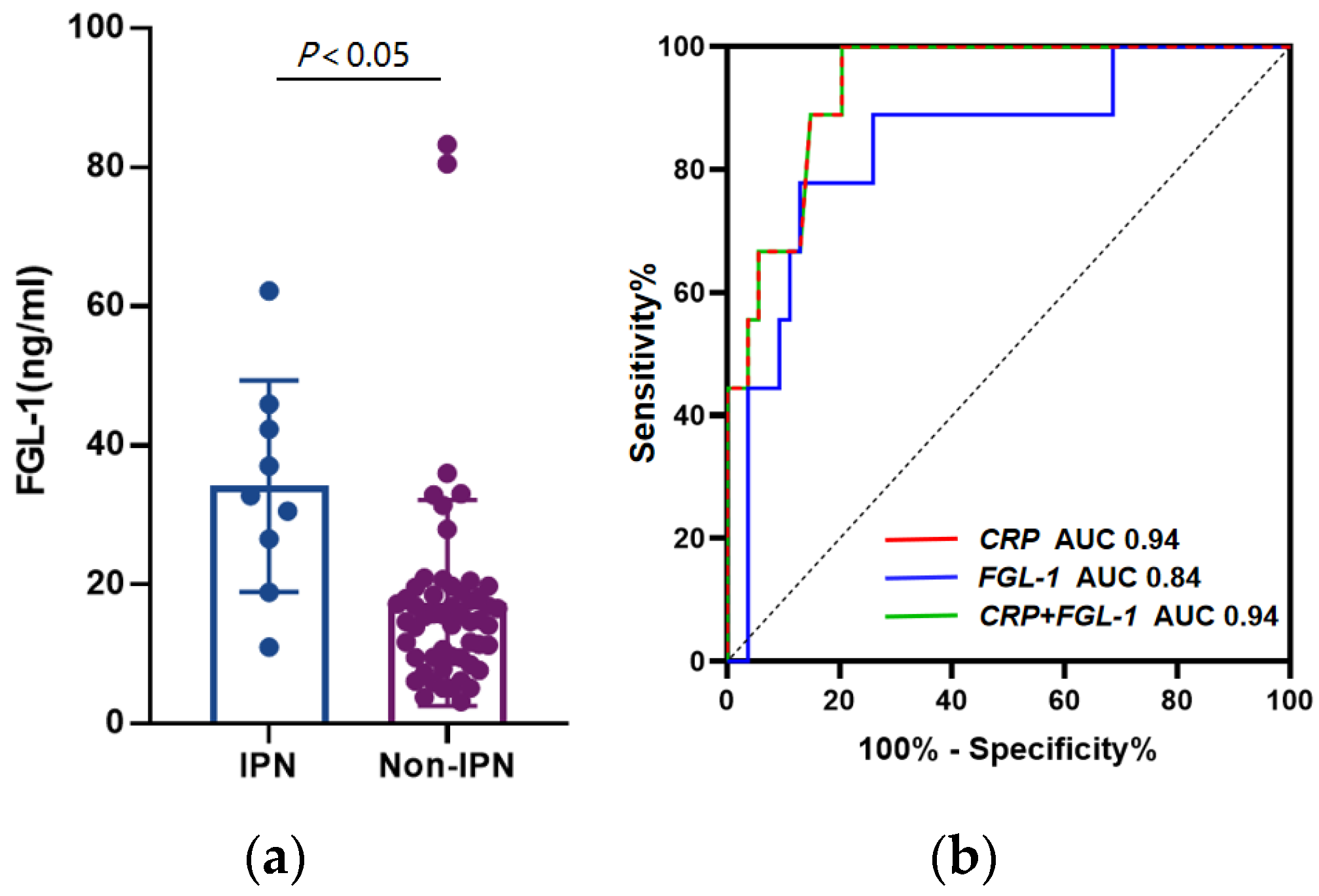
3.3. FGL-1 and CRP Shows Better Diagnostic Power in IPN
3.4. Combination of FGL-1 and PCT Improves the Predictive Capability for SAP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Banks, P.A.; Bollen, T.L.; Dervenis, C.; Gooszen, H.G.; Johnson, C.D.; Sarr, M.G.; Tsiotos, G.G.; Vege, S.S. Acute Pancreatitis Classification Working Group. Classification of acute pancreatitis—2012: Revision of the Atlanta classification and definitions by international consensus. Gut 2013, 62, 102–111. [Google Scholar] [CrossRef]
- Lazzarin, G.; Romano, L.; Coletti, G.; Di Sibio, A.; Vicentini, V.; Fatayer, M.W.A.; Schietroma, M.; Pessia, B.; Leone, M.; Carlei, F.; et al. Branch Duct—IPMN and PanIN, in IgG4-Autoimmune pancreatitis: A case report. Clin. Case Rep. 2020, 10, 2111–2115. [Google Scholar] [CrossRef]
- Lodewijkx, P.J.; Besselink, M.G.; Witteman, B.J.; Schepers, N.J.; Gooszen, H.G.; van Santvoort, H.C.; Bakker, O.J.; Dutch Pancreatitis Study Group. Nutrition in acute pancreatitis: A critical review. Expert Rev. Gastroenterol. Hepatol. 2016, 10, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Gukovskaya, A.S.; Gukovsky, I.; Algül, H.; Habtezion, A. Autophagy, inflammation, and immune dysfunction in the pathogenesis of pancreatitis. Gastroenterology 2017, 153, 1212–1226. [Google Scholar] [CrossRef] [Green Version]
- Kylänpää, M.L.; Repo, H.; Puolakkainen, P.A. Inflammation and immunosuppression in severe acute pancreatitis. World J. Gastroenterol. 2010, 16, 2867–2872. [Google Scholar] [CrossRef]
- Matull, W.R.; Pereira, S.P.; O’Donohue, J.W. Biochemical markers of acute pancreatitis. J. Clin. Pathol. 2006, 59, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, A.K.; Meher, S.; Prakash, S.; Tiwary, S.K.; Singh, U.; Srivastava, A.; Dixit, V.K. Comparison of Ranson, Glasgow, MOSS, SIRS, BISAP, APACHE-II, CTSI Scores, IL-6, CRP, and Procalcitonin in Predicting Severity, Organ Failure, Pancreatic Necrosis, and Mortality in Acute Pancreatitis. HPB Surg. 2013, 2013, 367581. [Google Scholar] [CrossRef] [Green Version]
- Neoptolemos, J.P.; Kemppainen, E.A.; Mayer, J.M.; Fitzpatrick, J.M.; Raraty, M.G.; Slavin, J.; Beger, H.G.; Hietaranta, A.J.; Puolakkainen, P.A. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: A multicentre study. Lancet 2000, 355, 1955–1960. [Google Scholar] [CrossRef]
- Woo, S.M.; Noh, M.H.; Kim, B.G.; Hsing, C.T.; Han, J.S.; Ryu, S.H.; Seo, J.M.; Yoon, H.A.; Jang, J.S.; Choi, S.R.; et al. Comparison of serum procalcitonin with Ranson, APACHE-II, Glasgow and Balthazar CT severity index scores in predicting severity of acute pancreatitis. Korean J. Gastroenterol. 2011, 5, 831–837. [Google Scholar] [CrossRef] [Green Version]
- Isman, F.K.; Zulfikaroglu, B.; Isbilen, B.; Ozalp, N.; Ozmen, M.M.; Bilgic, I.; Koc, M. Copeptin is a predictive biomarker of severity in acute pancreatitis. Am. J. Emerg. Med. 2013, 31, 690–692. [Google Scholar] [CrossRef]
- Mofidi, R.; Suttie, S.A.; Patil, P.V.; Ogston, S.; Parks, R.W. The value of procalcitonin at predicting the severity of acute pancreatitis and development of infected pancreatic necrosis: Systematic review. Surgery 2009, 146, 72–81. [Google Scholar] [CrossRef]
- Wu, H.T.; Chen, S.C.; Fan, K.C.; Kuo, C.H.; Lin, S.Y.; Wang, S.H.; Chang, C.J.; Li, H.Y. Targeting fibrinogen-like protein 1 is a novel therapeutic strategy to combat obesity. FASEB J. 2020, 34, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ukomadu, C. Fibrinogen-like protein 1, a hepatocyte derived protein is an acute phase reactant. Biochem. Biophys. Res. Commun. 2008, 365, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Waldron, R.T.; Lugea, A.; Gulla, A.; Pandol, S.J. Proteomic Identification of Novel Plasma Biomarkers and Pathobiologic Pathways in Alcoholic Acute Pancreatitis. Front. Physiol. 2018, 9, 1215. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, M.; Brady, M.; Shokuhi, S.; Christmas, S.; Neoptolemos, J.P.; Slavin, J. Inflammatory mediators in acute pancreatitis. J. Pathol. 2000, 190, 117–125. [Google Scholar] [CrossRef]
- Bhatia, M.; Wong, F.L.; Cao, Y.; Lau, H.Y.; Huang, J.; Puneet, P.; Chevali, L. Pathophysiology of acute pancreatitis. Pancreatology 2005, 5, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Widdison, A.L.; Karanjia, N.D. Pancreatic infection complicating acute pancreatitis. Br. J. Surg. 1993, 80, 148–154. [Google Scholar] [CrossRef]
- Phillip, V.; Steiner, J.M.; Algül, H. Early phase of acute pancreatitis: Assessment and management. World J. Gastrointest. Pathophysiol. 2014, 5, 158–168. [Google Scholar] [CrossRef]
- Meher, S.; Mishra, T.S.; Sasmal, P.K.; Rath, S.; Sharma, R.; Rout, B.; Sahu, M.K. Role of Biomarkers in Diagnosis and Prognostic Evaluation of Acute Pancreatitis. J. Biomark. 2015, 2015, 519–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Zhan, Y.Q.; Yu, M.; Ge, C.H.; Li, C.Y.; Zhang, J.H.; Wang, X.H.; Ge, Z.Q.; Yang, X.M. Hepassocin activates the EGFR/ERK cascade and induces proliferation of L02 cells through the Src-dependent pathway. Cell Signal. 2014, 26, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Cao, C.Z.; Xu, W.X.; Cao, M.M.; Yang, F.; Dong, L.; Yu, M.; Zhan, Y.Q.; Gao, Y.B.; Li, W.; et al. Recombinant human hepassocin stimulates proliferation of hepatocytes in vivo and improves survival in rats with fulminant hepatic failure. Gut 2010, 59, 817–826. [Google Scholar] [CrossRef]
- Calvaruso, V. Hepassocin as a treatment for fulminant hepatic failure: Will it translate from rats to human? Gut 2010, 59, 709–710. [Google Scholar] [CrossRef]
- Hara, H.; Yoshimura, H.; Uchida, S.; Toyoda, Y.; Aoki, M.; Sakai, Y.; Morimoto, S.; Shiokawa, K. Molecular cloning and functional expression analysis of a cDNA for human hepassocin, a liver-specific protein with hepatocyte mitogenic activity. Biochim. Biophys. Acta 2001, 1520, 45–53. [Google Scholar] [CrossRef]
- Rijken, D.C.; Dirkx, S.P.; Luider, T.M.; Leebeek, F.W. Hepatocyte-derived fibrinogen-related protein-1 is associated with the fibrin matrix of a plasma clot. Biochem. Biophys. Res. Commun. 2006, 350, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, J.; Shen, J.; Du, F.; Wu, X.; Li, M.; Chen, Y.; Cho, C.H.; Li, X.; Xiao, Z.; et al. The role of Fibrinogen-like proteins in Cancer. Int. J. Biol. Sci. 2021, 17, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Qi, L.W.; Alolga, R.N.; Liu, Q. Implication of the hepatokine, fibrinogen-like protein 1 in liver diseases, metabolic disorders and cancer: The need to harness its full potential. Int. J. Biol. Sci. 2022, 18, 292–300. [Google Scholar] [CrossRef]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W.; et al. Fibrinogen-like Protein 1 Is a Major Immune Inhibitory Ligand of LAG-3. Cell 2019, 176, 334–347. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Guo, Y.; Lu, L.; Lu, J.; Ke, M.; Xu, T.; Lu, Y.; Chen, W.; Wang, J.; Kong, D.; et al. Fibrinogen-Like Protein 1 Is a Novel Biomarker for Predicting Disease Activity and Prognosis of Rheumatoid Arthritis. Front. Immunol. 2020, 11, 579228. [Google Scholar] [CrossRef]
- Sun, X.L.; Qiao, L.C.; Gong, J.; Wen, K.; Xu, Z.Z.; Yang, B.L. Proteomics identifies a novel role of fibrinogen-like protein 1 in Crohn’s disease. World J. Gastroenterol. 2021, 27, 5946–5957. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Wang, G.; Li, L.; Hu, J.S.; Ji, L.; Li, Y.L.; Tian, F.Y.; Sun, B. Plasma D-Dimer Level Is an Early Predictor of Severity of Acute Pancreatitis Based on 2012 Atlanta Classification. Med. Sci. Monit. 2019, 25, 9019–9027. [Google Scholar] [CrossRef] [PubMed]
- Rau, B.M.; Kemppainen, E.A.; Gumbs, A.A.; Büchler, M.W.; Wegscheider, K.; Bassi, C.; Puolakkainen, P.A.; Beger, H.G. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): A prospective international multicenter study. Ann. Surg. 2007, 245, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.H.; Chen, T.Z.; Huai, J.P.; Lu, G.R.; Zhuge, X.J.; Chen, R.P.; Chen, W.J.; Wang, C.; Huang, Z.M. Correlation of fibrinogen-like protein 2 with progression of acute pancreatitis in rats. World J. Gastroenterol. 2013, 19, 2492–2500. [Google Scholar] [CrossRef] [PubMed]

| SAP (n = 12) | No SAP (n = 51) | p-Value | |
|---|---|---|---|
| Age—years | 50.5 (39.8–62.0) | 49.0 (41.0–64.0) | 0.951 |
| Gender—M/F | 8/4 | 31/20 | 0.706 |
| Comorbidities—no. (%) | |||
| Heart disease | 1/12 (8.33%) | 9/51 (17.65%) | 0.427 |
| Diabetes | 2/12 (16.67%) | 14/51 (27.45%) | 0.440 |
| Hypertension | 4/12 (33.33%) | 13/51 (25.49%) | 0.582 |
| hLOS—days | 26.0 (10.5–34.8) | 8.0 (6.0–12.0) | <0.001 |
| Etiology—no. (%) | |||
| Gallstones | 4/12 (33.33%) | 28/51 (54.90%) | 0.327 |
| Alcohol | 2/12 (16.67%) | 4/51 (7.84%) | 0.349 |
| Hypertriglyceridemia | 5/12 (41.67%) | 17/51 (33.33%) | 0.586 |
| Other | 1/12 (8.33%) | 2/51 (3.92%) | 0.518 |
| Smoking history—no. (%) | 4/12 (33.33%) | 12/39 (30.77%) | 0.483 |
| IPN (n = 9) | No IPN (n = 54) | p-Value | |
|---|---|---|---|
| Age—years | 44.0 (39.0–56.0) | 49.0 (41.0–64.0) | 0.298 |
| Gender—M/F | 7/2 | 32/22 | 0.290 |
| Comorbidities—no. (%) | |||
| Heart disease | 0/9 (0%) | 9/54 (16.67%) | 0.225 |
| Diabetes | 3/9 (33.33%) | 13/54 (24.07%) | 0.555 |
| Hypertension | 2/9 (22.22%) | 15/54 (27.78%) | 0.728 |
| hLOS—days | 18.5 (33.0–50.0) | 8.0 (5.75–11.25) | <0.001 |
| Etiology—no. (%) | |||
| Gallstones | 3/9 (33.33%) | 29/54 (53.70%) | 0.258 |
| Alcohol | 1/9 (11.11%) | 5/54 (9.26%) | 0.861 |
| Hypertriglyceridemia | 4/9 (44.44%) | 18/54 (33.33%) | 0.517 |
| Other | 1/9 (11.11%) | 2/54 (3.70%) | 0.334 |
| Smoking history—no. (%) | 2/9 (22.22%) | 14/54 (25.93%) | 0.910 |
| Severity | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n = 63 | SAP (n = 12) | No-SAP (n = 51) | X2 | p-Value | OR | 95% CI | p-Value |
| WBC (109/mL) | 0.459 | 0.549 | - | - | - | |||
| ≤10 | 27 | 4 | 23 | |||||
| >10 | 36 | 8 | 28 | |||||
| HCT (%) | 0.165 | 0.685 | - | - | - | |||
| ≤45 | 45 | 8 | 37 | |||||
| >45 | 18 | 4 | 14 | |||||
| Blood glucose (mmol/L) | 0.932 | 0.334 | - | - | - | |||
| ≤7 | 19 | 5 | 14 | |||||
| >7 | 44 | 7 | 37 | |||||
| Ca2+ (mmol/L) | 1.281 | 0.285 | - | - | - | |||
| <2.25 | 42 | 10 | 32 | |||||
| ≥2.25 | 21 | 2 | 19 | |||||
| FIB (g/L) | 0.466 | 0.496 | - | - | - | |||
| ≤4.66 | 37 | 6 | 31 | |||||
| >4.66 | 26 | 6 | 20 | |||||
| APTT (s) | 0.046 | 0.830 | - | - | - | |||
| ≤27.2 | 28 | 5 | 23 | |||||
| >27.2 | 35 | 7 | 28 | |||||
| PCT (ng/mL) | 9.908 | 0.002 | 0.047 | 0.002~0.991 | 0.049 | |||
| ≤0.5 | 31 | 1 | 30 | |||||
| >0.5 | 32 | 11 | 21 | |||||
| CRP (mg/dL) | 4.632 | 0.031 | 2.241 | 0.165~30.384 | 0.544 | |||
| ≤150 | 28 | 2 | 26 | |||||
| >150 | 35 | 10 | 25 | |||||
| FGL-1 (ng/mL) | 32.029 | <0.001 | 0.013 | 0.001~0.157 | 0.001 | |||
| ≤23.78 | 49 | 2 | 47 | |||||
| >23.78 | 14 | 10 | 4 | |||||
| IPN | Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n = 63 | IPN (n = 9) | No-IPN (n = 54) | X2 | p-Value | OR | 95% CI | p-Value |
| WBC (109/mL) | 0.389 | 0.533 | - | - | - | |||
| ≤10 | 27 | 3 | 24 | |||||
| >10 | 36 | 6 | 30 | |||||
| HCT (%) | 0.117 | 0.733 | - | - | - | |||
| ≤45 | 45 | 6 | 39 | |||||
| >45 | 18 | 3 | 15 | |||||
| Blood glucose (mmol/L) | 0.050 | 0.823 | - | - | - | |||
| ≤7 | 19 | 3 | 16 | |||||
| >7 | 44 | 6 | 38 | |||||
| Ca2+ (mmol/L) | 2.333 | 0.127 | - | - | - | |||
| <2.25 | 42 | 8 | 34 | |||||
| ≥2.25 | 21 | 1 | 20 | |||||
| FIB (g/L) | 0.884 | 0.347 | - | - | - | |||
| ≤4.66 | 37 | 4 | 33 | |||||
| >4.66 | 26 | 5 | 21 | |||||
| APTT (s) | 0.525 | 0.469 | - | - | - | |||
| ≤27.2 | 28 | 3 | 25 | |||||
| >27.2 | 35 | 6 | 29 | |||||
| PCT (ng/mL) | 14.860 | <0.001 | 0.447 | 0.038~5.264 | 0.522 | |||
| ≤3.5 | 57 | 5 | 52 | |||||
| >3.5 | 6 | 4 | 2 | |||||
| CRP (mg/dL) | 13.585 | <0.001 | 0.060 | 0.006~0.649 | 0.021 | |||
| ≤430 | 50 | 3 | 47 | |||||
| >430 | 13 | 6 | 7 | |||||
| FGL-1 (ng/mL) | 20.935 | <0.001 | 0.034 | 0.003~0.384 | 0.006 | |||
| ≤23.78 | 50 | 2 | 48 | |||||
| >23.78 | 13 | 7 | 6 | |||||
| AUC (95% CI) | Cut-Off | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | |
|---|---|---|---|---|---|---|---|---|
| PCT (ng/mL) | 0.89 (0.77–1.00) | 1.26 | 83.33% | 88.24% | 62.50% | 95.74% | 7.09 | 0.19 |
| FGL-1 (ng/mL) | 0.88 (0.75–1.00) | 23.78 | 83.33% | 94.12% | 76.92% | 96.00% | 19.23 | 0.24 |
| FGL-1 + PCT | 0.96 (0.88–1.00) | - | 91.67% | 98.04% | 91.67% | 98.04% | 46.77 | 0.08 |
| AUC (95% CI) | Cut-Off | Sensitivity | Specificity | PPV | NPV | LR+ | LR− | |
|---|---|---|---|---|---|---|---|---|
| CRP (mg/dL) | 0.94 (0.87–1.00) | 397 | 100% | 79.63% | 45.00% | 100% | 4.91 | 0 |
| FGL-1 (ng/mL) | 0.84 (0.70–0.99) | 23.79 | 77.78% | 87.04% | 50.00% | 95.92% | 6.00 | 0.26 |
| FGL-1 + CRP | 0.94 (0.87–1.00) | - | 100% | 79.63% | 91.67% | 98.04% | 4.91 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, Y.; Zhao, Z.; Zhang, Y.; Zhang, T.; Li, G.; Liu, L.; Tan, H.; Sun, B.; Li, L. Fibrinogen-like Protein 1 as a Predictive Marker for the Incidence of Severe Acute Pancreatitis and Infectious Pancreatic Necrosis. Medicina 2022, 58, 1753. https://doi.org/10.3390/medicina58121753
Sui Y, Zhao Z, Zhang Y, Zhang T, Li G, Liu L, Tan H, Sun B, Li L. Fibrinogen-like Protein 1 as a Predictive Marker for the Incidence of Severe Acute Pancreatitis and Infectious Pancreatic Necrosis. Medicina. 2022; 58(12):1753. https://doi.org/10.3390/medicina58121753
Chicago/Turabian StyleSui, Yuhang, Zhongjie Zhao, Yang Zhang, Tao Zhang, Guanqun Li, Liwei Liu, Hongtao Tan, Bei Sun, and Le Li. 2022. "Fibrinogen-like Protein 1 as a Predictive Marker for the Incidence of Severe Acute Pancreatitis and Infectious Pancreatic Necrosis" Medicina 58, no. 12: 1753. https://doi.org/10.3390/medicina58121753
APA StyleSui, Y., Zhao, Z., Zhang, Y., Zhang, T., Li, G., Liu, L., Tan, H., Sun, B., & Li, L. (2022). Fibrinogen-like Protein 1 as a Predictive Marker for the Incidence of Severe Acute Pancreatitis and Infectious Pancreatic Necrosis. Medicina, 58(12), 1753. https://doi.org/10.3390/medicina58121753






