Safety of Rigid Bronchoscopy for Therapeutic Intervention at the Intensive Care Unit Bedside
Abstract
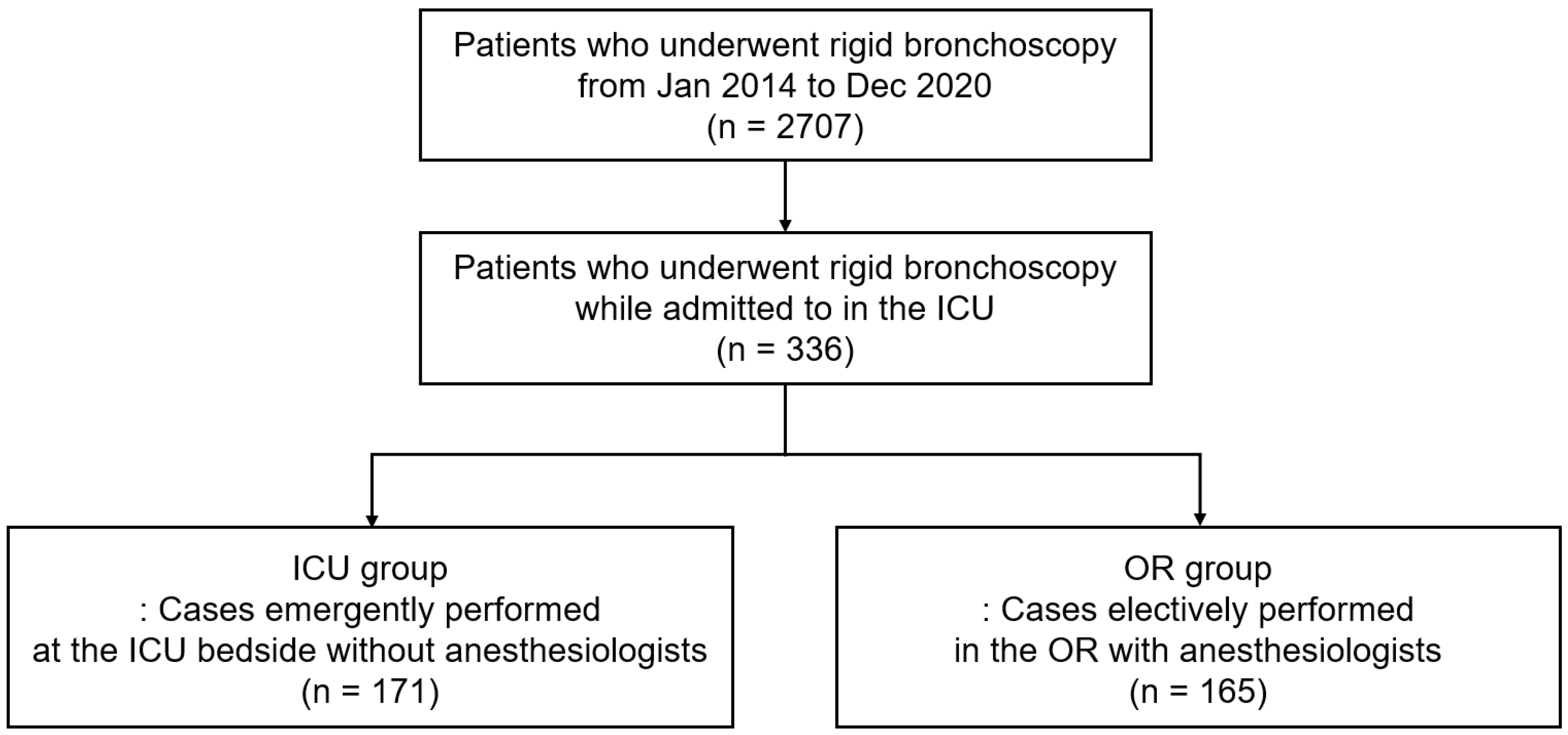
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anesthesia and Airway Intervention Techniques
2.3. Adverse Events
2.4. Data Collection
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Details of Rigid Bronchoscopy and Anesthetic Drugs
3.3. Procedure-Related Complications and Other Outcomes
3.4. Risk of Procedure-Related Complications
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Panchabhai, T.S.; Mehta, A.C. Historical perspectives of bronchoscopy. Connecting the dots. Ann. Am. Thorac. Soc. 2015, 12, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Batra, H.; Yarmus, L. Indications and complications of rigid bronchoscopy. Expert Rev. Respir. Med. 2018, 12, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.; Welsby, I.; Mahmood, K.; Wahidi, M.; MacIntyre, N.; Shofer, S. Ventilation and anesthetic approaches for rigid bronchoscopy. Ann. Am. Thorac. Soc. 2014, 11, 628–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lima, A.; Kheir, F.; Majid, A.; Pawlowski, J. Anesthesia for interventional pulmonology procedures: A review of advanced diagnostic and therapeutic bronchoscopy. Can. J. Anaesth. 2018, 65, 822–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahidi, M.M.; Shojaee, S.; Lamb, C.R.; Ost, D.; Maldonado, F.; Eapen, G.; Caroff, D.A.; Stevens, M.P.; Ouellette, D.R.; Lilly, C. The use of bronchoscopy during the coronavirus disease 2019 pandemic: CHEST/AABIP guideline and expert panel report. Chest 2020, 158, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Galway, U.; Zura, A.; Khanna, S.; Wang, M.; Turan, A.; Ruetzler, K. Anesthetic considerations for bronchoscopic procedures: A narrative review based on the Cleveland Clinic experience. J. Thorac. Dis. 2019, 11, 3156–3170. [Google Scholar] [CrossRef]
- Murgu, S.; Laxmanan, B.; Stoy, S.; Egressy, K.; Chaddha, U.; Farooqui, F.; Brunner, R.; Hogarth, K.; Chaney, M. Evaluation of Safety and Short-term Outcomes of Therapeutic Rigid Bronchoscopy Using Total Intravenous Anesthesia and Spontaneous Assisted Ventilation. Respiration 2020, 99, 239–247. [Google Scholar] [CrossRef]
- Chaddha, U.; Murgu, S. Complications of rigid bronchoscopy. Respirology 2021, 26, 14–18. [Google Scholar] [CrossRef]
- Jung, H.; Ko, R.E.; Ko, M.G.; Jeon, K. Trends of in-hospital cardiac arrests in a single tertiary hospital with a mature rapid response system. PLoS ONE 2022, 17, e0262541. [Google Scholar] [CrossRef]
- Na, S.J.; Ko, R.E.; Ko, M.G.; Jeon, K. Automated alert and activation of medical emergency team using early warning score. J. Intensive Care 2021, 9, 73. [Google Scholar] [CrossRef]
- Kim, H. Stenting therapy for stenosing airway disease. Respirology 1998, 3, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Chang, B.; Kim, H.; Jeong, B.-H. Interventional bronchoscopy in malignant central airway obstruction by extra-pulmonary malignancy. BMC Pulm. Med. 2018, 18, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, T.E.F.; Pearse, R.M.; Archbold, R.A.; Ahmad, T.; Niebrzegowska, E.; Wragg, A.; Rodseth, R.N.; Devereaux, P.J.; Ackland, G.L. A Prospective International Multicentre Cohort Study of Intraoperative Heart Rate and Systolic Blood Pressure and Myocardial Injury After Noncardiac Surgery: Results of the VISION Study. Anesth. Analg. 2018, 126, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Cortegiani, A.; Gregoretti, C.; Neto, A.; Hemmes, S.; Ball, L.; Canet, J.; Hiesmayr, M.; Hollmann, M.; Mills, G.; Melo, M. Association between night-time surgery and occurrence of intraoperative adverse events and postoperative pulmonary complications. Br. J. Anaesth. 2019, 122, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Südfeld, S.; Brechnitz, S.; Wagner, J.Y.; Reese, P.C.; Pinnschmidt, H.O.; Reuter, D.A.; Saugel, B. Post-induction hypotension and early intraoperative hypotension associated with general anaesthesia. Br. J. Anaesth. 2017, 119, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Hallqvist, L.; Granath, F.; Fored, M.; Bell, M. Intraoperative Hypotension and Myocardial Infarction Development Among High-Risk Patients Undergoing Noncardiac Surgery: A Nested Case-Control Study. Anesth. Analg. 2021, 133, 6–15. [Google Scholar] [CrossRef]
- Schultz, M.J.; Hemmes, S.N.; Neto, A.S.; Binnekade, J.M.; Canet, J.; Hedenstierna, G.; Jaber, S.; Hiesmayr, M.; Hollmann, M.W.; Mills, G.H. Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS-an observational study in 29 countries. Eur. J. Anaesthesiol. 2017, 34, 492–507. [Google Scholar]
- Baigent, C.; Blackwell, L.; Collins, R.; Emberson, J.; Godwin, J.; Peto, R.; Buring, J.; Hennekens, C.; Kearney, P.; Meade, T.; et al. Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009, 373, 1849–1860. [Google Scholar]
- Mayhew, D.; Mendonca, V.; Murthy, B.V.S. A review of ASA physical status—Historical perspectives and modern developments. Anaesthesia 2019, 74, 373–379. [Google Scholar] [CrossRef] [Green Version]
- Myer III, C.M.; O’Connor, D.M.; Cotton, R.T. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann. Otol. Rhinol. Laryngol. 1994, 103, 319–323. [Google Scholar] [CrossRef]
- Vannucci, A.; Riordan, I.R.; Prifti, K.; Sebastiani, A.; Helsten, D.L.; Lander, D.P.; Kallogjeri, D.; Cavallone, L. Prolonged time to extubation after general anaesthesia is associated with early escalation of care: A retrospective observational study. Eur. J. Anaesthesiol. 2021, 38, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Dutau, H.; Vandemoortele, T.; Breen, D.P. Rigid bronchoscopy. Clin. Chest Med. 2013, 34, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Dumon, J.F. A dedicated tracheobronchial stent. Chest 1990, 97, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Jeong, B.H.; Ng, J.; Jeong, S.H.; Kim, H. Clinical Outcomes of Complications Following Self-Expandable Metallic Stent Insertion for Benign Tracheobronchial Stenosis. Medicina 2020, 56, 367. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Yarmus, L.; Rendina, E.A.; Rafeq, S.; Andrade, R.; Michaud, G.; Kazakov, J.; Arias, S.; Ciccone, A.M.; Ortiz, R.; et al. Multi-institutional retrospective analysis of adverse events following rigid tracheobronchoscopy. Respirology 2021, 26, 87–91. [Google Scholar] [CrossRef]
- Dalar, L.; Özdemir, C.; Abul, Y.; Karasulu, L.; Sökücü, S.N.; Akbaş, A.; Altn, S. Therapeutic bronchoscopic interventions for malignant airway obstruction: A retrospective study from experience on 547 patients. Medicina 2016, 95, e3886. [Google Scholar] [CrossRef]
- Hermes, A.; Heigener, D.; Gatzemeier, U.; Schatz, J.; Reck, M. Efficacy and safety of bronchoscopic laser therapy in patients with tracheal and bronchial obstruction: A retrospective single institution report. Clin. Respir. J. 2012, 6, 67–71. [Google Scholar] [CrossRef]
- Ost, D.E.; Ernst, A.; Grosu, H.B.; Lei, X.; Diaz-Mendoza, J.; Slade, M.; Gildea, T.R.; Machuzak, M.; Jimenez, C.A.; Toth, J. Complications following therapeutic bronchoscopy for malignant central airway obstruction: Results of the AQuIRE registry. Chest 2015, 148, 450–471. [Google Scholar] [CrossRef] [Green Version]
- Ghojazadeh, M.; Sanaie, S.; Paknezhad, S.P.; Faghih, S.-S.; Soleimanpour, H. Using Ketamine and Propofol for Procedural Sedation of Adults in the Emergency Department: A Systematic Review and Meta-Analysis. Adv. Pharm. Bull. 2019, 9, 5–11. [Google Scholar] [CrossRef]

| Variables | Total (n = 336) | ICU Group (n = 171) | OR Group (n = 165) | p |
|---|---|---|---|---|
| Age, years | 63 (50–74) | 63 (50–74) | 63 (50–74) | 0.651 |
| Male | 158 (47.0) | 87 (50.9) | 71 (43.0) | 0.150 |
| BMI, kg/m2 | 21.1 (18.6–24.1) | 22.0 (19.3–24.3) | 20.8 (18.1–23.9) | 0.025 |
| Current or past smoker | 138 (41.1) | 77 (45.0) | 61 (37.0) | 0.133 |
| Comorbidities | ||||
| Cancer | 141 (42.0) | 69 (40.4) | 72 (43.6) | 0.542 |
| Diabetes mellitus | 97 (28.9) | 48 (28.1) | 49 (29.7) | 0.742 |
| Cerebrovascular disease | 57 (17.0) | 26 (15.2) | 31 (18.8) | 0.382 |
| Congestive heart failure | 53 (15.8) | 29 (17.0) | 24 (14.5) | 0.544 |
| Chronic pulmonary disease | 28 (8.3) | 17 (9.9) | 11 (6.7) | 0.278 |
| Chronic liver disease | 18 (5.4) | 9 (5.3) | 9 (5.5) | 0.938 |
| Performance status a | 0.714 | |||
| ASA III | 42 (12.5) | 20 (11.7) | 22 (13.3) | |
| ASA IV | 119 (35.4) | 64 (37.4) | 55 (33.3) | |
| ASA V | 175 (52.1) | 87 (50.9) | 88 (53.3) | |
| Tracheostomy before the intervention | 50 (14.9) | 23 (13.5) | 27 (16.4) | 0.453 |
| Invasive respiratory support before intervention | 170 (50.6) | 84 (49.1) | 86 (52.1) | 0.583 |
| Mechanical ventilation without ECMO | 155 (46.1) | 71 (41.5) | 84 (50.9) | 0.084 |
| With ECMO | 15 (4.5) | 13 (7.6) | 2 (1.2) | 0.005 |
| Arterial blood gas analysis b | ||||
| PaO2/FiO2 ratio, mmHg | 332 (241–445) | 287 (206–391) | 370 (272–463) | <0.001 |
| <200 and/or ECMO | 66 (19.6) | 48 (28.1) | 18 (10.9) | |
| 200–299 | 78 (23.2) | 41 (24.0) | 37 (22.4) | <0.001 c |
| ≥300 and/or no results | 192 (57.1) | 82 (48.0) | 110 (66.7) | |
| PaCO2, mmHg | 38.5 (33.2–43.9) | 38.0 (32.7–44.5) | 38.9 (34.1–43.6) | 0.684 |
| HCO3, mEq/L | 25.0 (22.3–28.1) | 24.6 (22.1–27.5) | 25.4 (22.8–28.4) | 0.119 |
| Reason for intervention | ||||
| PITS | 132 (39.3) | 59 (34.5) | 73 (44.2) | 0.068 |
| MCAO | 116 (34.5) | 59 (34.5) | 57 (34.5) | 0.993 |
| POTS | 24 (7.1) | 12 (7.0) | 12 (7.3) | 0.928 |
| Airway FB | 20 (6.0) | 19 (11.1) | 1 (0.6) | <0.001 |
| Relapsing polychondritis | 17 (5.1) | 8 (4.7) | 9 (5.5) | 0.746 |
| PTBS | 14 (4.2) | 9 (5.3) | 5 (3.0) | 0.306 |
| Others d | 13 (3.9) | 5 (2.9) | 8 (4.8) | 0.360 |
| Variables | Total (n = 336) | ICU Group (n = 171) | OR Group (n = 165) | p |
|---|---|---|---|---|
| Time interval from ICU admission to intervention, hours | 24 (14–60) | 18 (3–36) | 41 (19–70) | <0.001 |
| <3 h | 41 (12.2) | 39 (22.8) | 2 (1.2) | <0.001 |
| <6 h | 62 (18.5) | 59 (34.5) | 3 (1.8) | <0.001 |
| Time of intervention | ||||
| Weekends or nighttime a | 86 (25.6) | 84 (49.1) | 2 (1.2) | <0.001 |
| Weekdays outside of the elective time b | 66 (19.6) | 64 (37.4) | 2 (1.2) | <0.001 |
| Weekdays in the elective time b | 184 (54.8) | 23 (13.5) | 161 (97.6) | <0.001 |
| Site of lesion | ||||
| Single lesion | 251 (74.7) | 123 (71.9) | 128 (77.6) | 0.234 c |
| Trachea | 205 (61.0) | 97 (56.7) | 108 (65.5) | 0.101 |
| RMB and/or RBI | 23 (6.8) | 10 (5.8) | 13 (7.9) | 0.461 |
| LMB | 17 (5.1) | 12 (7.0) | 5 (3.0) | 0.095 |
| Lobar bronchus | 6 (1.8) | 4 (2.3) | 2 (1.2) | 0.685 |
| Extended lesion | 85 (25.3) | 48 (28.1) | 37 (22.4) | 0.234 c |
| Trachea and any bronchi | 63 (18.8) | 37 (21.6) | 26 (15.8) | 0.167 |
| Both main bronchi | 20 (6.0) | 11 (6.4) | 9 (5.5) | 0.705 |
| One main bronchus and contralateral lobar bronchus | 2 (0.6) | 0 | 2 (1.2) | 0.240 |
| Severity of stenosis d | 0.014 | |||
| II | 117 (34.8) | 49 (28.7) | 68 (41.2) | |
| III | 138 (41.1) | 71 (41.5) | 67 (40.6) | |
| IV | 81 (24.1) | 51 (29.8) | 30 (18.2) | |
| Procedure details | ||||
| Stent insertion | 221 (65.8) | 108 (63.2) | 113 (68.5) | 0.304 |
| Stent change or reposition | 75 (22.3) | 30 (17.5) | 45 (27.3) | 0.032 |
| Tumor removal | 57 (17.0) | 30 (17.5) | 27 (16.4) | 0.773 |
| Stent removal | 32 (9.5) | 19 (11.1) | 13 (7.9) | 0.313 |
| Bougienation only e | 28 (8.3) | 9 (5.3) | 19 (11.5) | 0.038 |
| Tracheostomy | 25 (7.4) | 12 (7.0) | 13 (7.9) | 0.764 |
| Foreign body removal | 20 (6.0) | 19 (11.1) | 1 (0.6) | <0.001 |
| Laser cauterization | 12 (3.6) | 0 | 12 (7.3) | <0.001 |
| EBV insertion | 3 (0.9) | 3 (1.8) | 0 | 0.248 |
| Anesthetic agents | ||||
| Sedative | ||||
| Midazolam | 142 (42.3) | 78 (45.6) | 64 (38.8) | 0.205 |
| Ketamine | 95 (28.3) | 94 (55.0) | 1 (0.6) | <0.001 |
| Propofol | 181 (53.9) | 19 (11.1) | 162 (98.2) | <0.001 |
| Opioid | ||||
| Fentanyl | 149 (44.3) | 146 (85.4) | 3 (1.8) | <0.001 |
| Remifentanil | 164 (48.8) | 4 (2.3) | 160 (97.0) | <0.001 |
| NMBA | ||||
| Vecuronium | 23 (6.8) | 22 (12.9) | 1 (0.6) | <0.001 |
| Cisatracurim | 26 (7.7) | 25 (14.6) | 1 (0.6) | <0.001 |
| Succinylcholine | 8 (2.4) | 3 (1.8) | 5 (3.0) | 0.443 |
| Rocuronium | 275 (81.8) | 118 (69.0) | 157 (95.2) | <0.001 |
| Reversal agents for NMBA | 131 (39.0) | 2 (1.2) | 129 (78.2) | <0.001 |
| Sugammadex | 119 (35.4) | 2 (1.2) | 117 (70.9) | <0.001 |
| Neostigmine and glycopyrrolate | 12 (3.6) | 0 (0) | 12 (7.3) | <0.001 |
| Intervention duration, minutes | 17 (12–27) | 20 (12–30) | 16 (12–22) | 0.008 |
| Time interval from the end of intervention to the first extubation, minutes f | 85 (15–349) | 168 (62–1002) | 18 (8–124) | <0.001 |
| ≤15 min | 80 (23.8) | 7 (4.1) | 73 (44.2) | |
| 16–60 min | 63 (18.8) | 32 (18.7) | 31 (18.8) | <0.001 g |
| ≥61 min | 193 (57.4) | 132 (77.2) | 61 (37.0) |
| Variables | Total (n = 336) | ICU Group (n = 171) | OR Group (n = 165) | p |
|---|---|---|---|---|
| Intra-procedural complication | 280 (83.3) | 147 (86.0) | 133 (80.6) | 0.188 |
| Hypertension | 150 (44.6) | 103 (60.2) | 47 (28.5) | <0.001 |
| Hypotension | 107 (31.8) | 19 (11.1) | 88 (53.3) | <0.001 |
| Tachycardia | 164 (48.8) | 104 (60.8) | 60 (36.4) | <0.001 |
| Hypoxia | 37 (11.0) | 31 (18.1) | 6 (3.6) | <0.001 |
| Severe intra-procedural complication | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Post-procedural complication | 61 (18.2) | 41 (24.0) | 20 (12.1) | 0.005 |
| Respiratory failure | 23 (6.8) | 15 (8.8) | 8 (4.8) | 0.155 |
| Atelectasis | 15 (4.5) | 11 (6.4) | 4 (2.4) | 0.075 |
| Pneumonia | 12 (3.6) | 7 (4.1) | 5 (3.0) | 0.600 |
| Pleural effusion | 6 (1.8) | 3 (1.8) | 3 (1.8) | 1.000 |
| Newly developed arrhythmia | 5 (1.5) | 4 (2.3) | 1 (0.6) | 0.372 |
| Major bleeding | 4 (1.2) | 3 (1.8) | 1 (0.6) | 0.623 |
| Pneumothorax | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Severe post-procedural complication | 26 (7.7) | 18 (10.5) | 8 (4.8) | 0.052 |
| Severe respiratory failure | 23 (6.8) | 15 (8.8) | 8 (4.8) | 0.155 |
| Severe atelectasis | 2 (0.6) | 2 (1.2) | 0 (0.0) | 0.499 |
| Severe pneumonia | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Severe pleural effusion | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Severe newly developed arrhythmia | 1 (0.3) | 1 (0.6) | 0 (0.0) | 1.000 |
| Severe major bleeding | 1 (0.3) | 1 (0.6) | 0 (0.0) | 1.000 |
| Severe pneumothorax | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Lengths of ICU stay after intervention, day | 4 (3–6) | 3 (2–6) | 4 (3–6) | 0.147 |
| Need for additional intervention | 61 (18.2) | 30 (17.5) | 31 (18.8) | 0.767 |
| Mortality | ||||
| Procedure-related mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| ICU mortality | 16 (4.8) | 10 (5.8) | 6 (3.6) | 0.341 |
| Model | Intra-Procedural Complications | Post-Procedural Complications | Severe Post-Procedural Complications | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | Odds Ratio (95% CI) | p | |
| Crude | 1.47 (0.83–2.63) | 0.189 | 2.29 (1.27–4.10) | 0.006 | 2.31 (0.98–5.47) | 0.057 |
| Model 1 | 1.49 (0.71–3.13) | 0.295 | 2.82 (1.30–6.09) | 0.009 | 2.51 (0.75–8.40) | 0.136 |
| Model 2 | 1.72 (0.92–3.22) | 0.092 | 2.50 (1.37–4.55) | 0.003 | 2.68 (1.10–6.47) | 0.028 |
| Model 3 | 1.27 (0.69–2.34) | 0.434 | 2.34 (1.26–4.34) | 0.007 | 2.10 (0.86–5.13) | 0.105 |
| Model 4 | 1.44 (0.66–3.14) | 0.359 | 3.19 (1.43–7.11) | 0.005 | 2.54 (0.73–8.88) | 0.144 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Chang, B.; Ahn, H.J.; Kim, J.A.; Yang, M.; Kim, H.; Jeong, B.-H. Safety of Rigid Bronchoscopy for Therapeutic Intervention at the Intensive Care Unit Bedside. Medicina 2022, 58, 1762. https://doi.org/10.3390/medicina58121762
Kim SH, Chang B, Ahn HJ, Kim JA, Yang M, Kim H, Jeong B-H. Safety of Rigid Bronchoscopy for Therapeutic Intervention at the Intensive Care Unit Bedside. Medicina. 2022; 58(12):1762. https://doi.org/10.3390/medicina58121762
Chicago/Turabian StyleKim, Sang Hyuk, Boksoon Chang, Hyun Joo Ahn, Jie Ae Kim, Mikyung Yang, Hojoong Kim, and Byeong-Ho Jeong. 2022. "Safety of Rigid Bronchoscopy for Therapeutic Intervention at the Intensive Care Unit Bedside" Medicina 58, no. 12: 1762. https://doi.org/10.3390/medicina58121762







