Whole-Body Vibration or Aerobic Exercise in Patients with Bronchiectasis? A Randomized Controlled Study
Abstract
1. Introduction
2. Methods
2.1. Trial Design
2.2. Inclusion Criteria
2.3. Exclusion Criteria
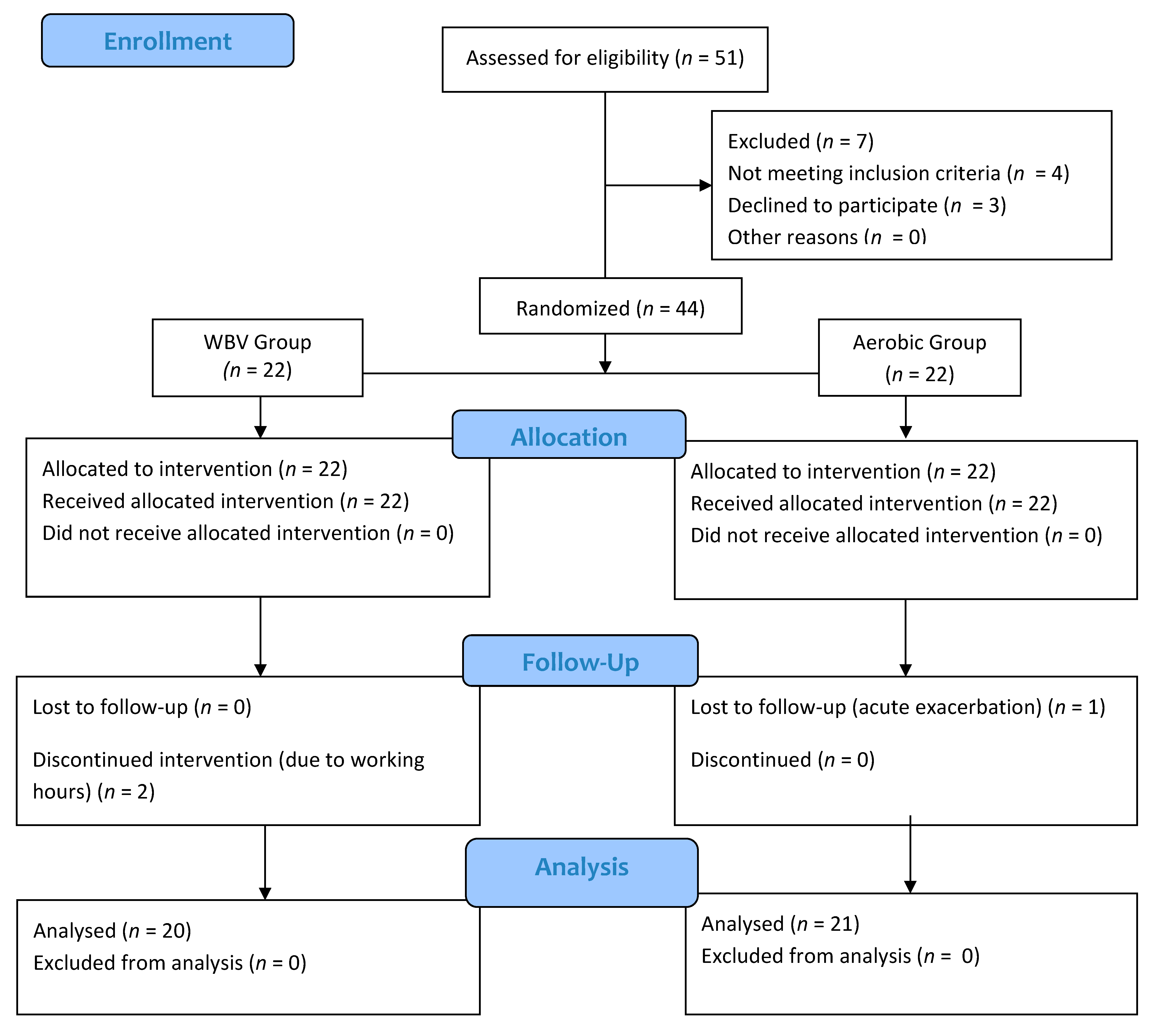
2.4. Randomization
2.5. Participants
3. Measurements
3.1. Evaluation
3.1.1. Pulmonary Function
3.1.2. Dyspnea
3.1.3. Exercise Capacity
3.1.4. Quality of Life
4. Intervention
4.1. Aerobic Exercise
4.2. Vibration Training
5. Sample Calculation
6. Analysis
7. Results
8. Discussion
Limitations of the Study
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, A.T.; Sullivan, A.L.; Chalmers, J.D. British Thoracic Society Guideline for bronchiectasis in adults. Thorax 2019, 74 (Suppl. S1), 1–69. [Google Scholar] [CrossRef]
- Jones, A.; Rowe, B.H. Bronchopulmonary hygiene physical therapy in bronchiectasis and chronic obstructive pulmonary disease: A systematic review. Heart Lung 2000, 29, 125–135. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.; O’Donnell, A.E.; Bradley, J.M. Airway clearance, muco-active therapies and pulmonary rehabilitation in bronchiectasis. Respirology 2019, 24, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Cole, A.D.; Nolan, C.M.; Barker, R.E.; Jones, S.E.; Kon, S.; Cairn, J.; Loebinger, M.; Wilson, R.; Man, W.D.-C. Pulmonary rehabilitation in bronchiectasis: A propensity-matched study. Eur. Respir. J. 2019, 53, 1801264. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos, E.; Pérez, M.E.; Guirao, L.; Samitier, B.; Costea, M.; Ortega, P.; González, M.V.; del Carmen, V.A.; Ovejero, L.; Moreno, E.; et al. Effects of whole body vibration training in patients with severe chronic obstructive. Respirology 2013, 18, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Harwood, B.; Scherer, J.; Brown, R.E.; Cornett, K.M.D.; Kenno, K.A.; Jakobi, J.M. Neuromuscular responses of the plantar flexors to whole-body vibration. Scand. J. Med. Sci. Sport. 2017, 27, 1569–1575. [Google Scholar]
- Shirato, R.; Sakamoto, H.; Sugiyama, T. Inhibitory Effects of Prolonged Vibratory Stimulus on the Maximal Voluntary Contraction Force and Muscle Activity of the Triceps Brachii: An Experimental Study. J. Chiropr. Med. 2019, 18, 97–105. [Google Scholar] [CrossRef]
- Costantino, C.; Bertuletti, S.; Romiti, D. Efficacy of whole-body vibration board training on strength in athletes after anterior cruciate ligament reconstruction: A randomized controlled study. Clin. J. Sport Med. 2018, 28, 339–349. [Google Scholar] [CrossRef]
- Furness, T.; Maschette, W. Influence of whole body vibration platform frequency on neuromuscular performance of community-dwelling older adults. J. Strength Cond. Res. 2009, 23, 1508–1513. [Google Scholar] [CrossRef]
- Rittweger, J.; Schiessl, H.; Felsenberg, D. Oxygen uptake during whole-body vibration exercise: Comparison with squatting as a slow voluntary movement. Eur. J. Appl. Physiol. 2001, 86, 169–173. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, Y.; Wang, P.; He, C.; He, H. Effects of whole body vibration on pulmonary function, functional exercise capacity and quality of life in people with chronic obstructive pulmonary disease: A systematic review. Clin. Rehabil. 2016, 30, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Braz Júnior, D.S.; de Andrade, A.D.; Teixeira, A.S.; Cavalcanti, C.A.; Morais, A.B.; Marinho, P.E. Whole-body vibration improves functional capacity and quality of life in patients with severe chronic obstructive pulmonary disease (copd): A pilot study. Int. J. Chronic Obstr. Pulm. Dis. 2015, 10, 125–132. [Google Scholar]
- Gloeckl, R.; Heinzelmann, I.; Baeuerle, S.; Damm, E.; Schwedhelm, A.-L.; Diril, M.; Buhrow, D.; Jerrentrup, A.; Kenn, K. Effects of whole body vibration in patients with chronic obstructive pulmonary disease—A randomized controlled trial. Respir. Med. 2012, 106, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rittweger, J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur. J. Appl. Physiol. 2010, 108, 877–904. [Google Scholar] [CrossRef] [PubMed]
- De Sire, A.; Lippi, L.; Ammendolia, A.; Cisari, C.; Venetis, K.; Sajjadi, E.; Fusco, N.; Invernizzi, M. Physical Exercise with or without Whole-Body Vibration in Breast Cancer Patients Suffering from Aromatase Inhibitor—Induced Musculoskeletal Symptoms: A Pilot Randomized Clinical Study. J. Pers. Med. 2021, 11, 1369. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Frederico, É.H.; Guimarães, C.A.; Reis-Silva, A.; de Oliveira Guedes-Aguiar, E.; Francisca Santos, A.; Cristina Moura-Fernandes, M.; Felipe Ferreira-Souza, L.; Eduardo-Santos, T.; Eduardo-Santos, D.; et al. Biological Effects of Paullinia cupana (Guarana) in Combination with Whole-Body Vibration Exercise in Wistar Rats. Appl. Sci. 2020, 10, 1104. [Google Scholar] [CrossRef]
- Rietschel, E.; van Koningsbruggen, S.; Fricke, O.; Semler, O.; Schoenau, E. Whole body vibration: A new therapeutic approach to improve muscle function in cystic fibrosis? Int. J. Rehabil. Res. 2008, 31, 253–256. [Google Scholar] [CrossRef]
- Jackson, K.; Merriman, H.; Vanderburgh, P.; Brahler, C.J. Acute effects of whole-body vibration on lower extremity muscle performance in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2008, 32, 171–176. [Google Scholar] [CrossRef]
- Nasrallah, T.M.; Emam, H.; Alwakil, I.M. Effects of Whole-Body Vibration on Egyptian Patients with Chronic Obstructive Pulmonary Disease. Int. J. Med. Arts 2020, 2, 420–426. [Google Scholar] [CrossRef]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef]
- Crisafulli, E.; Clini, E.M. Measures of dyspnea in pulmonary rehabilitation. Multidiscip. Resp. Med. 2010, 5, 202. [Google Scholar] [CrossRef] [PubMed]
- Özalevli, S.; Uçan, E.S. Farklı dispne skalalarının kronik obstrüktif akciğer hastalığında karşılaştırılması. Toraks Derg. 2004, 5, 90–94. [Google Scholar]
- Patel, S.; Barker, R.; Walsh, J.; Polgar, O.; Nolan, C.; Man, W. The five-repetition sit-to-stand test (5STS) in patients with bronchiectasis: Validity and responsiveness. Eur. Respir. J. 2020, 56 (Suppl. S64), 1830. [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Polatlı, M.; Yorgancıoğlu, A.; Aydemir, Ö.; Demirci, N.Y.; Kirkil, G.; Nayci, S.A.; Köktürk, N.; Uysal, A.; Akdemir, S.E.; Özgür, E.S.; et al. St. George Solunum Anketinin Türkçe Geçerlilik Ve Güvenilirliği. Tuberk Toraks 2013, 61, 81–87. [Google Scholar] [CrossRef]
- Wilson, C.B.; Jones, P.W.; O’Leary, C.J.; Cole, P.J.; Wilson, R. Validation of the St. George’s Respiratory Questionnaire in bronchiectasis. Am. J. Respir. Crit. Care Med. 1997, 156, 536–541. [Google Scholar] [CrossRef]
- Mehri, S.N.; Khoshnevis, M.A.; Zarrehbinan, F.; Hafezi, S.; Ghasemi, A.; Ebadi, A. Effect of treadmill exercise training on VO2 peak in chronic obstructive pulmonary disease. Tanaffos 2007, 6, 18–24. [Google Scholar]
- Nito, M.; Yoshimoto, T.; Hashizume, W. Vibration decreases the responsiveness of Ia afferents and spinal motoneurons in humans. J. Neurophysiol. 2021, 126, 1137–1147. [Google Scholar] [CrossRef]
- Alam, M.M.; Khan, A.A.; Farooq, M. Effect of whole-body vibration on neuromuscular performance: A literature review. Work 2018, 59, 571–583. [Google Scholar] [CrossRef]
- Furness, T.; Bate, N.; Welsh, L.; Naughton, G.; Lorenzen, C. Efficacy of a whole-body vibration intervention to effect exercise tolerance and functional performance of the lower limbs of people with chronic obstructive pulmonary disease. BMC Pulm. Med. 2012, 12, 71. [Google Scholar] [CrossRef]
- Greulich, T.; Nell, C.; Koepke, J.; Fechtel, J.; Franke, M.; Schmeck, B.; Haid, D.; Apelt, S.; Filipovic, S.; Kenn, K.; et al. Benefits of whole body vibration training in patients hospitalised for COPD exacerbations-a randomized clinical trial. BMC Pulm. Med. 2014, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Puhan, M.A.; Mador, M.J.; Held, U.; Goldstein, R.; Guyatt, G.H.; Schünemann, H.J. Interpretation of treatment changes in 6-minute walk distance in patients with COPD. Eur. Respir. J. 2008, 32, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Lage, V.K.; Lacerda, A.C.R.; Neves, C.D.; Chaves, M.G.A.; Soares, A.A.; Lima, L.P.; Matos, M.A.; Leite, H.R.; Fernandes, J.S.C.; Oliveira, V.C.; et al. Cardiorespiratory responses in different types of squats and frequencies of whole body vibration in patients with chronic obstructive pulmonary disease. J. Appl. Physiol. 2019, 126, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sañudo, B.; Seixas, A.; Gloeckl, R.; Rittweger, J.; Rawer, R.; Taiar, R.; van der Zee, E.A.; van Heuvelen, M.J.G.; Lacerda, A.C.; Sartorio, A.; et al. Potential application of whole body vibration exercise for improving the clinical conditions of COVID-19 infected individuals: A narrative review from the world association of vibration exercise experts (wavex) panel. Int. J. Environ. Res. Public Health 2020, 17, 3650. [Google Scholar] [CrossRef] [PubMed]
- Sá-Caputo, D.C.; Coelho-Oliveira, A.C.; Pessanha-Freitas, J.; Paineiras-Domingos, L.L.; Lacerda, A.C.R.; Mendonça, V.A.; Sonza, A.; Taiar, R.; Sartorio, A.; Seixas, A.; et al. Whole-Body Vibration Exercise: A Possible Intervention in the Management of Post COVID-19 Complications? Appl. Sci. 2021, 11, 5733. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Padilla, J.; Moras, G.; Haro, C.L.; Fernández-Solà, J. Six weeks of whole-body vibration exercise improves pain and fatigue in women with fibromyalgia. J. Altern. Complement. Med. 2008, 14, 975–981. [Google Scholar] [CrossRef]
- Vaidya, T.; Chambellan, A.; de Bisschop, C. Sit-to-stand tests for COPD: A literature review. Respir. Med. 2017, 128, 70–77. [Google Scholar] [CrossRef]
- Furness, T.; Joseph, C.; Naughton, G.; Welsh, L.; Lorenzen, C. Benefits of whole-body vibration to people with copd: A community-based effi-cacy trial. BMC Pulm. Med. 2014, 14, 38. [Google Scholar] [CrossRef]
- Ora, J.; Prendi, E.; Ritondo, B.L.; Pata, X.; Spada, F.; Rogliani, P. Pulmonary Rehabilitation in Noncystic Fibrosis Bronchiectasis. Respiration 2022, 101, 97–105. [Google Scholar] [CrossRef]
- Hsieh, M.-J.; Lan, C.-C.; Chen, N.-H.; Huang, C.-C.; Wu, Y.-H.; Cho, H.-Y.; Tsai, Y.-H. Effects of high-intensity exercise training in a pulmonary rehabilitation programme for patients with chronic obstructive pulmonary disease. Respirology 2007, 12, 381–388. [Google Scholar] [CrossRef]
- Corlateanu, A.; Botnaru, V.; Covantev, S.; Dumitru, S.; Siafakas, N. Predicting health-related quality of life in patients with chronic obstructive pulmonary disease: The impact of age. Respiration 2016, 92, 229–234. [Google Scholar] [CrossRef] [PubMed]
| Variables | WBV Group (n = 20) X ± SD | Aerobic Exercise Group (n = 21) X ± SD | Z | p Value |
|---|---|---|---|---|
| Age (years) | 55.66 ± 17.10 | 41,66 ± 15.41 | −1.949 | 0.051 |
| Height (m) | 163 ± 9.58 | 164 ± 7.6 | −0.581 | 0.561 |
| Weight (kg) | 71.1 ± 10.64 | 61.55 ± 5.98 | −1.990 | 0.047 * |
| BMI (kg/m2) | 26.71 ± 3.29 | 22.96 ± 2.91 | −2.033 | 0.400 |
| Smoking history (pack years) | 4.22 ± 4.17 | 3.11 ± 4.91 | −0.584 | 0.559 |
| Number of exacerbations per year (n) | 1.55 ± 0.88 | 0.77 ± 0.66 | −1.927 | 0.054 |
| Disease severity a | ||||
| Mild bronchiectasis (0–4) | 3 ± 1.14 | 2.75 ± 1.25 | −0.139 | 0.889 |
| Moderate bronchiectasis (5–8) | 6 ± 1.15 | 6.66 ± 1.54 | −0.727 | 0.467 |
| Severe bronchiectasis (9+) | 9.66 ± 1.15 | 9.5 ± 0.7 | −1.291 | 0.197 |
| Disease Duration (years) | 16.44 ± 13.67 | 25.33 ± 15.16 | −1.552 | 0.121 |
| mMRC | 2.55 ± 1.01 | 2.44 ± 2.06 | −0.142 | 0.887 |
| FEV1 (L) | 1.59 ± 0.44 | 1.93 ± 0.46 | −1.372 | 0.170 |
| FEV1 (%) | 58 ± 14.23 | 59.44 ± 12.78 | −0.310 | 0.757 |
| FVC (L) | 2.25 ± 0.6 | 2.53 ± 0.53 | −0.884 | 0.377 |
| FVC (%) | 66.88 ± 14.17 | 69.22 ± 11.36 | −0.623 | 0.533 |
| FEV1/FVC (%) | 70.33 ± 8.21 | 71.88 ± 8.1 | −0.399 | 0.690 |
| PEF (L/sn) | 3.89 ± 1.83 | 4.46 ± 1.46 | −0.833 | 0.377 |
| PEF (%) | 50.76 ± 16.68 | 57.55 ± 12.88 | −0.708 | 0.479 |
| 6 MWT (m) | 433 ± 67.61 | 432.88 ± 96.22 | −0.132 | 0.895 |
| FTSST (seconds) | 11.75 ± 3.75 | 10.85 ± 2.6 | −0.574 | 0.566 |
| Saint georges respiratory questionnaire | ||||
| Total | 49.88 ± 13.18 | 54.21 ± 18.93 | −0.662 | 0.508 |
| Activity | 58.39 ± 26.64 | 66.72 ± 17 | −0.487 | 0.626 |
| Symptom | 53.06 ± 18.07 | 61.1 ± 22.38 | −0.751 | 0.453 |
| Impact | 44.02 ± 13.72 | 44.91 ± 24.02 | −0.397 | 0.691 |
| Variables | WBV Group (n = 20) | Aerobic Exercise Group (n = 21) | Differences between Groups | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline X ± SD | Post Treatment X ± SD | Z | pa | Baseline X ± SD | Post Treatment X ± SD | z | p a | z | p b | |
| FEV1 (L) | 1.59 ± 0.44 | 1.53 ± 0.45 | −0.178 | 0.858 | 1.93 ± 0.46 | 1.91 ± 0.41 | −0.421 | 0.674 | −1.680 | 0.093 |
| FEV1 (%) | 58 ± 14.23 | 56.32 ± 16.24 | −0.060 | 0.952 | 59.44 ± 12.78 | 61.77 ± 11.06 | −1.562 | 0.118 | −0.532 | 0.595 |
| FVC (L) | 2.25 ± 0.6 | 2.26 ± 0.6 | −0.420 | 0.674 | 2.53 ± 0.53 | 2.64 ± 0.58 | −2.207 | 0.027 * | −1.546 | 0.122 |
| FVC (%) | 66.88 ± 14.17 | 66.88 ± 14.15 | 0.000 | 1.000 | 69.22 ± 11.36 | 73.68 ± 8.57 | −2.207 | 0.027 * | −1.683 | 0.092 |
| FEV1/FVC (%) | 70.33 ± 8.21 | 68.44 ± 9.90 | −0.178 | 0.859 | 71.88 ± 8.1 | 72.98 ± 13.67 | −0.315 | 0.752 | −0.972 | 0.331 |
| PEF (L/sn) | 3.89 ± 1.83 | 4.07 ± 2.06 | −0.980 | 0.327 | 4.46 ± 1.46 | 4.84 ± 1.73 | −0.170 | 0.865 | −0.751 | 0.453 |
| PEF (%) | 50.76 ± 16.68 | 51.81 ± 15.37 | −0.416 | 0.677 | 57.55 ± 12.88 | 54.21 ± 18.93 | −1.272 | 0.203 | −1.679 | 0.093 |
| 6 MWT (m) | 433 ± 67.61 | 477.91 ± 41.21 | −2.310 | 0.021 * | 432.88 ± 96.22 | 473.85 ± 99.58 | −2.668 | 0.008 * | −0.132 | 0.895 |
| FTSST (seconds) | 11.75 ± 3.75 | 10.18 ± 3.23 | −2.429 | 0.015 * | 10.85 ± 2.6 | 9.19 ± 2.42 | −2.547 | 0.011 * | −0.927 | 0.354 |
| mMRC | 2.55 ± 1.01 | 0.78 ± 0.8 | −2.724 | 0.006 | 2.44 ± 2.06 | 3 ± 2.11 | −1.732 | 0.083 | −2.703 | 0.007 |
| SGRQ | ||||||||||
| Total | 49.88 ± 13.18 | 43.55 ± 14.61 | −0.700 | 0.484 | 54.21 ± 18.93 | 27.65 ± 9.82 | −2.666 | 0.008 * | −2.075 | 0.038 * |
| Activity | 58.39 ± 26.64 | 54.20 ± 16.47 | −0.420 | 0.674 | 66.72 ± 17 | 32.22 ± 14.23 | −2.666 | 0.008 * | −2.433 | 0.015 * |
| Symptom | 53.06 ± 18.07 | 48.33 ± 17.55 | −0.980 | 0.327 | 61.1 ± 22.38 | 44.32 ± 17.48 | −1.400 | 0.161 | −0.662 | 0.508 |
| Impact | 44.02 ± 13.72 | 35.98 ± 14.61 | −1.120 | 0.263 | 44.91 ± 24.02 | 19.82 ± 13.71 | −2.240 | 0.025 * | −1.898 | 0.058 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atalay, O.T.; Yılmaz, A.; Bahtiyar, B.C.; Altınışık, G. Whole-Body Vibration or Aerobic Exercise in Patients with Bronchiectasis? A Randomized Controlled Study. Medicina 2022, 58, 1790. https://doi.org/10.3390/medicina58121790
Atalay OT, Yılmaz A, Bahtiyar BC, Altınışık G. Whole-Body Vibration or Aerobic Exercise in Patients with Bronchiectasis? A Randomized Controlled Study. Medicina. 2022; 58(12):1790. https://doi.org/10.3390/medicina58121790
Chicago/Turabian StyleAtalay, Orçin Telli, Ayşenur Yılmaz, Betül Cengiz Bahtiyar, and Göksel Altınışık. 2022. "Whole-Body Vibration or Aerobic Exercise in Patients with Bronchiectasis? A Randomized Controlled Study" Medicina 58, no. 12: 1790. https://doi.org/10.3390/medicina58121790
APA StyleAtalay, O. T., Yılmaz, A., Bahtiyar, B. C., & Altınışık, G. (2022). Whole-Body Vibration or Aerobic Exercise in Patients with Bronchiectasis? A Randomized Controlled Study. Medicina, 58(12), 1790. https://doi.org/10.3390/medicina58121790






