Elevated Preoperative NMPR Predicts an Unfavorable Chance of Survival in Resectable Esophageal Squamous Cell Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Design
2.2. Data Collection and Definition
2.3. Statistical Analyses
3. Results
3.1. Clinicopathologic Characteristics
3.2. Associations between NMPR and Clinicopathologic Parameters
3.3. Associations between NMPR and Prognosis in Patients with Resectable ESCC
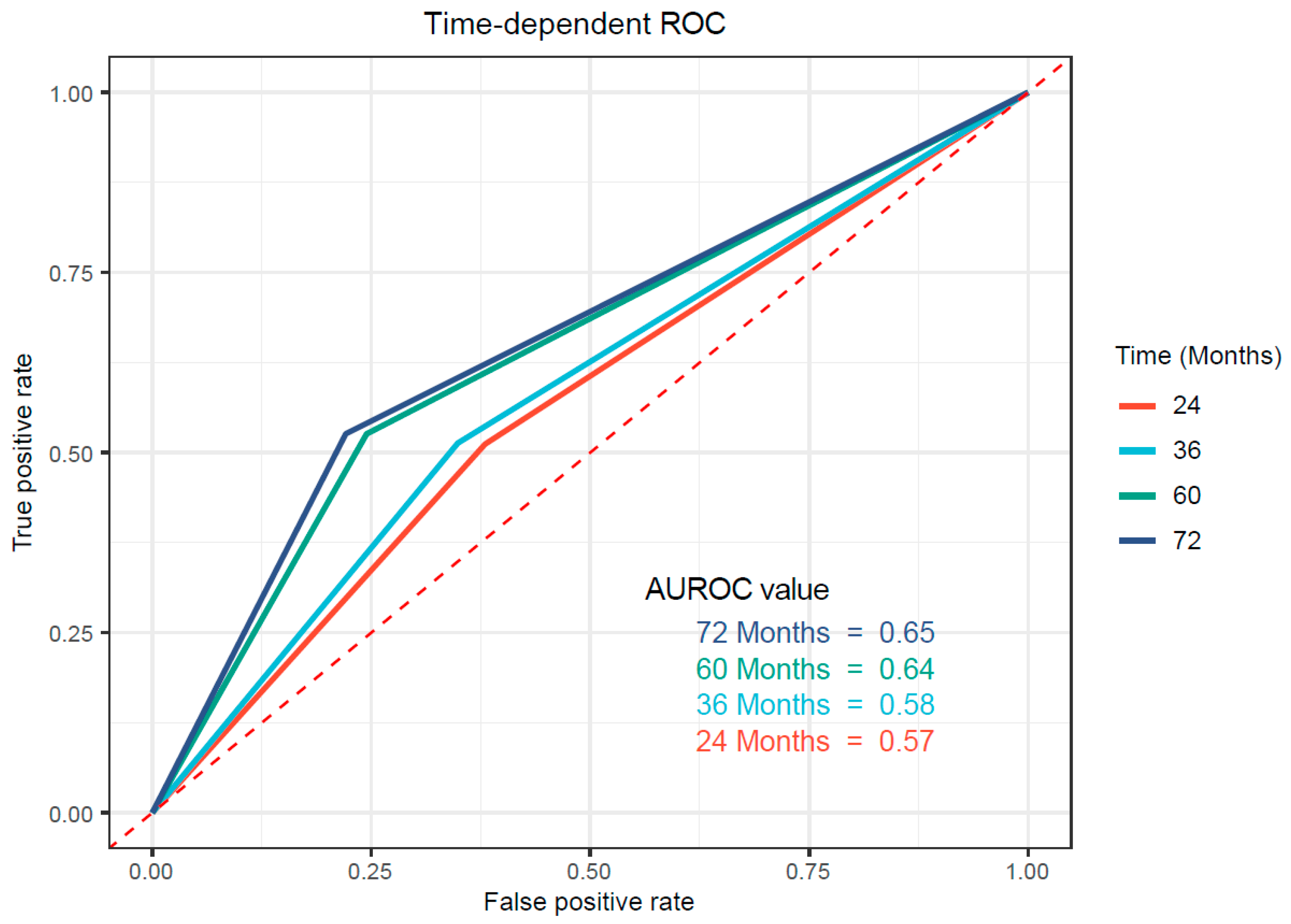
3.4. Validation of the Prognostic Value of NMPR in an External Cohort
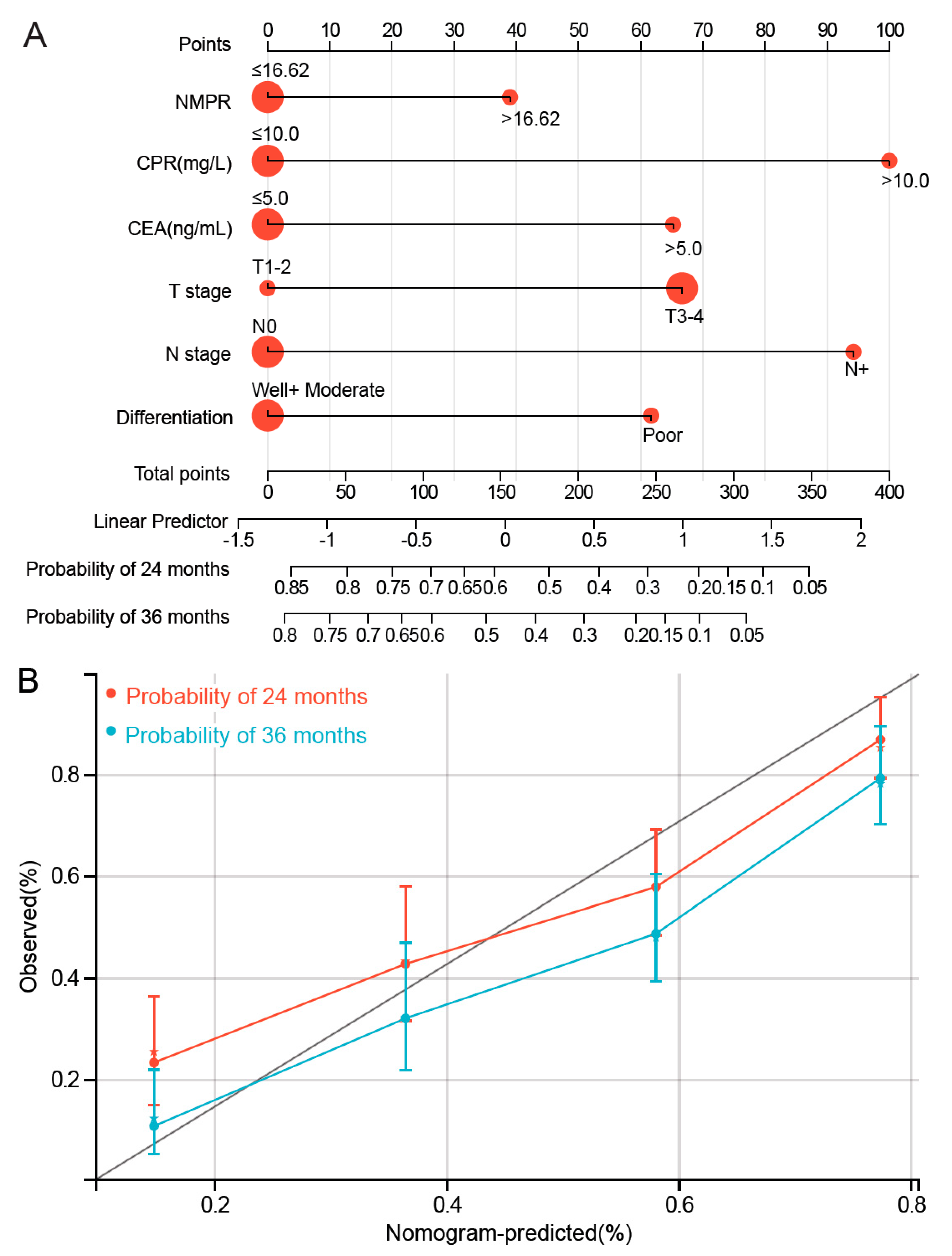
3.5. Development of NMPR-Based Nomogram for OS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar]
- Hu, J.; Kuang, P.; Chen, D.; Chen, Y.; Wen, Z. Prognostic significance of serum carcinoembryonic antigen and squamous cell carcinoma antigen in patients with esophageal squamous cell carcinoma undergoing radical esophagectomy. Transl. Cancer Res. 2020, 9, 2460–2471. [Google Scholar] [CrossRef] [PubMed]
- Yodying, H.; Matsuda, A.; Miyashita, M.; Matsumoto, S.; Sakurazawa, N.; Yamada, M.; Uchida, E. Prognostic Significance of Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Oncologic Outcomes of Esophageal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2016, 23, 646–654. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Xu, J.; Liu, X.; Zhen, Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm. Sin. B 2021, 11, 3379–3392. [Google Scholar] [CrossRef] [PubMed]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Smyth, E.C.; Lagergren, J.; Fitzgerald, R.C.; Lordick, F.; Shah, M.A.; Lagergren, P.; Cunningham, D. Oesophageal cancer. Nat. Rev. Dis. Primers 2017, 3, 17048. [Google Scholar] [CrossRef]
- Li, D.L.; Zhang, L.; Yan, H.J.; Zheng, Y.B.; Guo, X.G.; Tang, S.J.; Hu, H.Y.; Yan, H.; Qin, C.; Zhang, J.; et al. Machine learning models predict lymph node metastasis in patients with stage T1-T2 esophageal squamous cell carcinoma. Front Oncol. 2022, 12, 986358. [Google Scholar] [CrossRef]
- Xie, X.; Luo, K.J.; Hu, Y.; Wang, J.Y.; Chen, J. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis. Esophagus 2016, 29, 79–85. [Google Scholar] [CrossRef]
- Ravindranathan, D.; Master, V.A.; Bilen, M.A. Inflammatory Markers in Cancer Immunotherapy. Biology 2021, 10, 325. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Yang, C.; Li, X.; Pan, C.; Rao, J.; Li, N.; Liao, W.; Lin, L. Combined neutrophil/platelet/lymphocyte/differentiation score predicts chemosensitivity in advanced gastric cancer. BMC Cancer 2018, 18, 515. [Google Scholar] [CrossRef]
- Sun, S.Y.; Zhao, B.Q.; Wang, J.; Mo, Z.X.; Zhao, Y.N.; Wang, Y.; He, J. The clinical implications of mean platelet volume and mean platelet volume/platelet count ratio in locally advanced esophageal squamous cell carcinoma. Dis. Esophagus 2018, 31, dox125. [Google Scholar] [CrossRef] [PubMed]
- Dupre, A.; Malik, H.Z. Inflammation and cancer: What a surgical oncologist should know. Eur. J. Surg. Oncol 2018, 44, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, X.; Liu, J.; Chen, S.; Xu, D.; Li, W.; Zhan, Y.; Li, Y.; Chen, Y.; Zhou, Z. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio in predicting survival for patients with stage I-II gastric cancer. Chin. J. Cancer 2016, 35, 57. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Zhu, S.; Pan, H.; Cao, X.; Yuan, S.; Hu, X. Combined neutrophil-platelet score and hemoglobin level predict survival in esophageal squamous cell carcinoma patients treated with chemoradiotherapy. Oncotarget 2017, 8, 87971–87979. [Google Scholar] [CrossRef][Green Version]
- Zhu, S.; Miao, C.W.; Wang, Z.T.; Peng, L.; Li, B. Sensitivity value of hematological markers in patients receiving chemoradiotherapy for esophageal squamous cell carcinoma. OncoTargets Ther. 2016, 9, 6187–6193. [Google Scholar] [CrossRef][Green Version]
- Watt, D.G.; Proctor, M.J.; Park, J.H.; Horgan, P.G.; McMillan, D.C. The Neutrophil-Platelet Score (NPS) Predicts Survival in Primary Operable Colorectal Cancer and a Variety of Common Cancers. PLoS ONE 2015, 10, e142159. [Google Scholar] [CrossRef][Green Version]
- Feng, J.F.; Sheng, C.; Zhao, Q.; Chen, P. Prognostic value of mean platelet volume/platelet count ratio in patients with resectable esophageal squamous cell carcinoma: A retrospective study. PeerJ 2019, 7, e7246. [Google Scholar] [CrossRef]
- Di, Q.S.; Xu, T.; Song, Y.; Zuo, Z.G.; Cao, F.J.; Yu, X.J.; Tang, J.Y.; Zhang, W.; Li, C.; Wan, G.X.; et al. High C-Reactive Protein to Albumin Ratio Predicts Inferior Clinical Outcomes in Extranodal Natural Killer T-Cell Lymphoma. Dose Response 2020, 18, 710600640. [Google Scholar] [CrossRef]
- Sharma, T.; Gupta, A.; Chauhan, R.; Bhat, A.A.; Nisar, S.; Hashem, S.; Akhtar, S.; Ahmad, A.; Haris, M.; Singh, M.; et al. Cross-talk between the microbiome and chronic inflammation in esophageal cancer: Potential driver of oncogenesis. Cancer Metastasis Rev. 2022, 41, 281–299. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Lu, J.; Li, C.; Li, N. The prognostic value of systemic immune-inflammation index in surgical esophageal cancer patients: An updated meta-analysis. Front. Surg. 2022, 9, 922595. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Liu, Y.; Cheng, S.; Sun, Z.; Sheng, C.; Sun, X.; Shang, X.; Tian, W.; Wang, X.; Li, J.; et al. Diagnosis and survival values of neutrophil-lymphocyte ratio (NLR) and red blood cell distribution width (RDW) in esophageal cancer. Clin. Chim. Acta 2019, 488, 150–158. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, Y.; Cai, Z.; Lin, X. The prognostic and predictive value of the combination of the neutrophil-to-lymphocyte ratio and the platelet-to-lymphocyte ratio in patients with hepatocellular carcinoma who receive transarterial chemoembolization therapy. Cancer Manag. Res. 2019, 11, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Tazzyman, S.; Niaz, H.; Murdoch, C. Neutrophil-mediated tumour angiogenesis: Subversion of immune responses to promote tumour growth. Semin. Cancer Biol. 2013, 23, 149–158. [Google Scholar] [CrossRef]
- Huang, H.; Liu, Q.; Zhu, L.; Zhang, Y.; Lu, X.; Wu, Y.; Liu, L. Prognostic Value of Preoperative Systemic Immune-Inflammation Index in Patients with Cervical Cancer. Sci. Rep. 2019, 9, 3284. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in cancer carcinogenesis and metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, F.; He, Z.; Zuo, M.Z. Clinicopathological and prognostic significance of platelet count in patients with ovarian cancer. Climacteric 2018, 21, 60–68. [Google Scholar] [CrossRef]
- Ishii, Y.; Hamashima, T.; Yamamoto, S.; Sasahara, M. Pathogenetic significance and possibility as a therapeutic target of platelet derived growth factor. Pathol. Int. 2017, 67, 235–246. [Google Scholar] [CrossRef]
- Kamath, S.; Blann, A.D.; Lip, G.Y. Platelet activation: Assessment and quantification. Eur. Heart J. 2001, 22, 1561–1571. [Google Scholar] [CrossRef]
- Tokoro, T.; Makino, I.; Harada, S.; Okamoto, K.; Nakanuma, S.; Sakai, S.; Kinoshita, J.; Nakamura, K.; Miyashita, T.; Tajima, H.; et al. Interactions Between Neutrophils and Platelets in the Progression of Acute Pancreatitis. Pancreas 2020, 49, 830–836. [Google Scholar] [CrossRef]
- Liang, X.; Xiu, C.; Liu, M.; Lin, C.; Chen, H.; Bao, R.; Yang, S.; Yu, J. Platelet-neutrophil interaction aggravates vascular in fl ammation and promotes the progression of atherosclerosis by activating the TLR4/NF-kappaB pathway. J. Cell Biochem. 2019, 120, 5612–5619. [Google Scholar] [CrossRef] [PubMed]
- Lisman, T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018, 371, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Stoiber, D.; Assinger, A. Platelet-Leukocyte Interplay in Cancer Development and Progression. Cells 2020, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Abd, E.H.; Abdel, M.I.; El, H.R.; Meshaal, S.; Shimila, I.; Radwan, E.R. Potential Role of Neutrophil-Platelet Interaction in Increased Susceptibility to Infection of Patients with Down Syndrome. Lab. Med. 2022, 53, 405–411. [Google Scholar]
- Sreeramkumar, V.; Adrover, J.M.; Ballesteros, I.; Cuartero, M.I.; Rossaint, J.; Bilbao, I.; Nacher, M.; Pitaval, C.; Radovanovic, I.; Fukui, Y.; et al. Neutrophils scan for activated platelets to initiate inflammation. Science 2014, 346, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Zhu, J.; Wu, S.; Luo, H.; He, Y. Prognostic Significance of Platelet (PLT) and Platelet to Mean Platelet Volume (PLT/MPV) Ratio During Apatinib Second-Line or Late-Line Treatment in Advanced Esophageal Squamous Cell Carcinoma Patients. Technol. Cancer Res. Treat. 2022, 21, 2091152434. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.W.; Zhou, J.Y.; Jiang, W.; Ji, L.; Liu, Y.C.; Yin, X.X. The Combination of Preoperative Nutritional Risk Screening-2002 and Neutrophil-to-Lymphocyte Ratio is a Useful Prognostic Marker in Patients with Esophageal Squamous Cell Carcinoma. Nutr. Cancer 2021, 73, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, J.S.; Feng, J.F. The combination of preoperative serum C-reactive protein and carcinoembryonic antigen is a useful prognostic factor in patients with esophageal squamous cell carcinoma: A combined ROC analysis. OncoTargets Ther. 2015, 8, 795–803. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Wang, W.; Wang, S.; Hou, J.; Zhang, A.; Lv, B.; Gao, C.; Yan, Z.; Pang, D.; et al. Regulation of Hippo/YAP signaling and Esophageal Squamous Carcinoma progression by an E3 ubiquitin ligase PARK2. Theranostics 2020, 10, 9443–9457. [Google Scholar] [CrossRef]
| Characteristics | Total | NMPR of ZJ Cohort | p-Value | Total | NMPR of SY Cohort | p-Value | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| (N = 277) | ≤16.62 (n = 155) | >16.62 (n = 122) | (N = 101) | ≤16.62 (n = 60) | >16.62 (n = 41) | ||||
| Age (years) | 0.886 | 0.299 | 0.008 | ||||||
| ≤60 | 158 (57%) | 89 (56%) | 69 (44%) | 42 (42%) | 23 (55%) | 19 (45%) | |||
| >60 | 119 (43%) | 66 (55%) | 53 (45%) | 59 (58%) | 37 (63%) | 22 (37%) | |||
| Gender | 0.126 | 0.631 | 0.899 | ||||||
| Female | 37 (13%) | 25 (68%) | 12 (32%) | 14 (14%) | 9 (64%) | 5 (36%) | |||
| Male | 240 (87%) | 130 (54%) | 110 (46%) | 87 (86%) | 51 (59%) | 36 (41%) | |||
| Tumor length (cm) | 0.004 | 0.013 | 0.001 | ||||||
| ≤3.0 | 74 (27%) | 52 (70%) | 22 (30%) | 46 (46%) | 33 (72%) | 13 (28%) | |||
| >3.0 | 203 (73%) | 103 (51%) | 100 (49%) | 55 (54%) | 27 (49%) | 28 (51%) | |||
| Tumor location | 0.488 | 0.042 | <0.001 | ||||||
| Upper + Middle | 145 (52%) | 84 (58%) | 61 (42%) | 22 (22%) | 17 (77%) | 5 (23%) | |||
| Lower | 132 (48%) | 71 (54%) | 61 (46%) | 79 (78%) | 43 (54%) | 36 (46%) | |||
| Vessel invasion | 0.474 | 0.719 | <0.001 | ||||||
| Negative | 232 (84%) | 132 (57%) | 100 (43%) | 58 (57%) | 33 (57%) | 25 (43%) | |||
| Positive | 45 (16%) | 23 (51%) | 22 (49%) | 43 (43%) | 27 (63%) | 16 (37%) | |||
| Differentiation | 0.326 | 0.152 | 0.364 | ||||||
| Well+ Moderate | 223 (81%) | 128 (57%) | 95 (43%) | 77 (76%) | 48 (62%) | 29 (38%) | |||
| Poor | 54 (19%) | 27 (50%) | 27 (50%) | 24 (24%) | 12 (50%) | 12 (50%) | |||
| T stage | 0.055 | 0.002 | 0.491 | ||||||
| T1 + T2 | 99 (36%) | 63 (64%) | 36 (36%) | 40 (40%) | 31 (76%) | 9 (24%) | |||
| T3 + T4 | 178 (64%) | 92 (52%) | 86 (48%) | 61 (60%) | 29 (48%) | 32 (52%) | |||
| N stage | 0.616 | 0.338 | <0.001 | ||||||
| N0 | 150 (54%) | 86 (57%) | 64 (43%) | 77 (76%) | 47 (61%) | 30 (39%) | |||
| N+ | 127 (46%) | 69 (54%) | 58 (46%) | 24 (24%) | 13 (54%) | 11 (46%) | |||
| TNM stage | 0.337 | 0.312 | 0.004 | ||||||
| I + II | 161 (58%) | 94 (58%) | 67 (42%) | 75 (74%) | 46 (61%) | 29 (39%) | |||
| III | 116 (42%) | 61 (53%) | 55 (47%) | 26 (26%) | 14 (54%) | 12 (46%) | |||
| CEA (ng/mL) | 0.926 | ||||||||
| ≤5.0 | 239 (86%) | 134 (56%) | 105 (44%) | ||||||
| >5.0 | 38 (14%) | 21 (55%) | 17 (45%) | ||||||
| CRP (mg/L) | 0.028 | ||||||||
| ≤10.0 | 198 (71%) | 119 (60%) | 79 (40%) | ||||||
| >10.0 | 79 (29%) | 36 (46%) | 43 (54%) | ||||||
| Variable | Univariate | p-Value | Multivariate | p-Value | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age (≤60 y/>60 y) | 1.088 | 0.806–1.468 | 0.583 | |||
| Gender (Female/Male) | 1.392 | 0.854–2.268 | 0.185 | |||
| Tumor length (≤3 cm/>3 cm) | 1.767 | 1.224–2.553 | 0.002 | 1.106 | 0.734–1.667 | 0.630 |
| CRP (≤10 ng/mL/>10 ng/mL) | 2.258 | 1.652–3.083 | <0.001 | 2.383 | 1.718–3.307 | <0.001 |
| Tumor location (Upper + Middle/Lower) | 1.168 | 0.868–1.573 | 0.305 | |||
| Vessel invasion (Negative/Positive) | 1.725 | 1.194–2.492 | 0.004 | 0.986 | 0.669–1.453 | 0.942 |
| Differentiation (Well + Moderate/Poor) | 1.515 | 1.060–2.165 | 0.023 | 1.674 | 1.143–2.452 | 0.008 |
| T stage (T1-2/T3-4) | 2.170 | 1.541–3.057 | <0.001 | 1.718 | 1.168–2.528 | 0.006 |
| N stage (N0/N+) | 2.626 | 1.932–3.569 | <0.001 | 2.257 | 1.632–3.123 | <0.001 |
| CEA (≤5 mg/L/>5 mg/L) | 1.707 | 1.114–2.546 | 0.009 | 1.752 | 1.167–2.630 | 0.007 |
| Adjuvant therapy (No/Yes) | 1.291 | 0.944–1.766 | 0.110 | |||
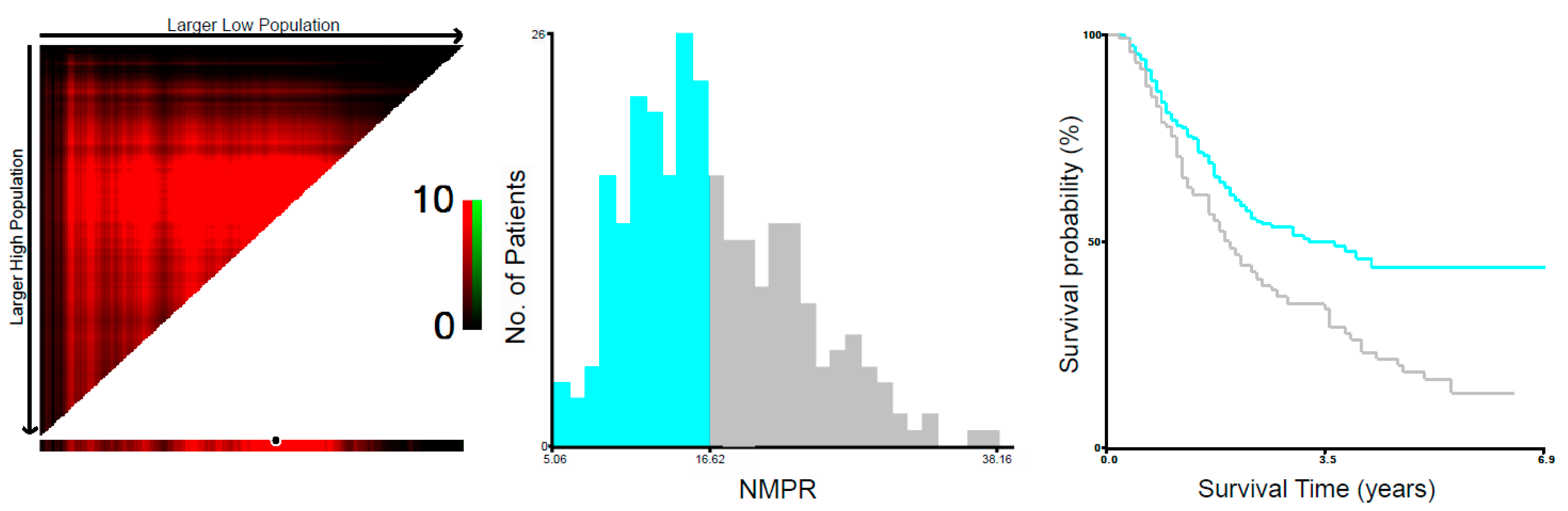
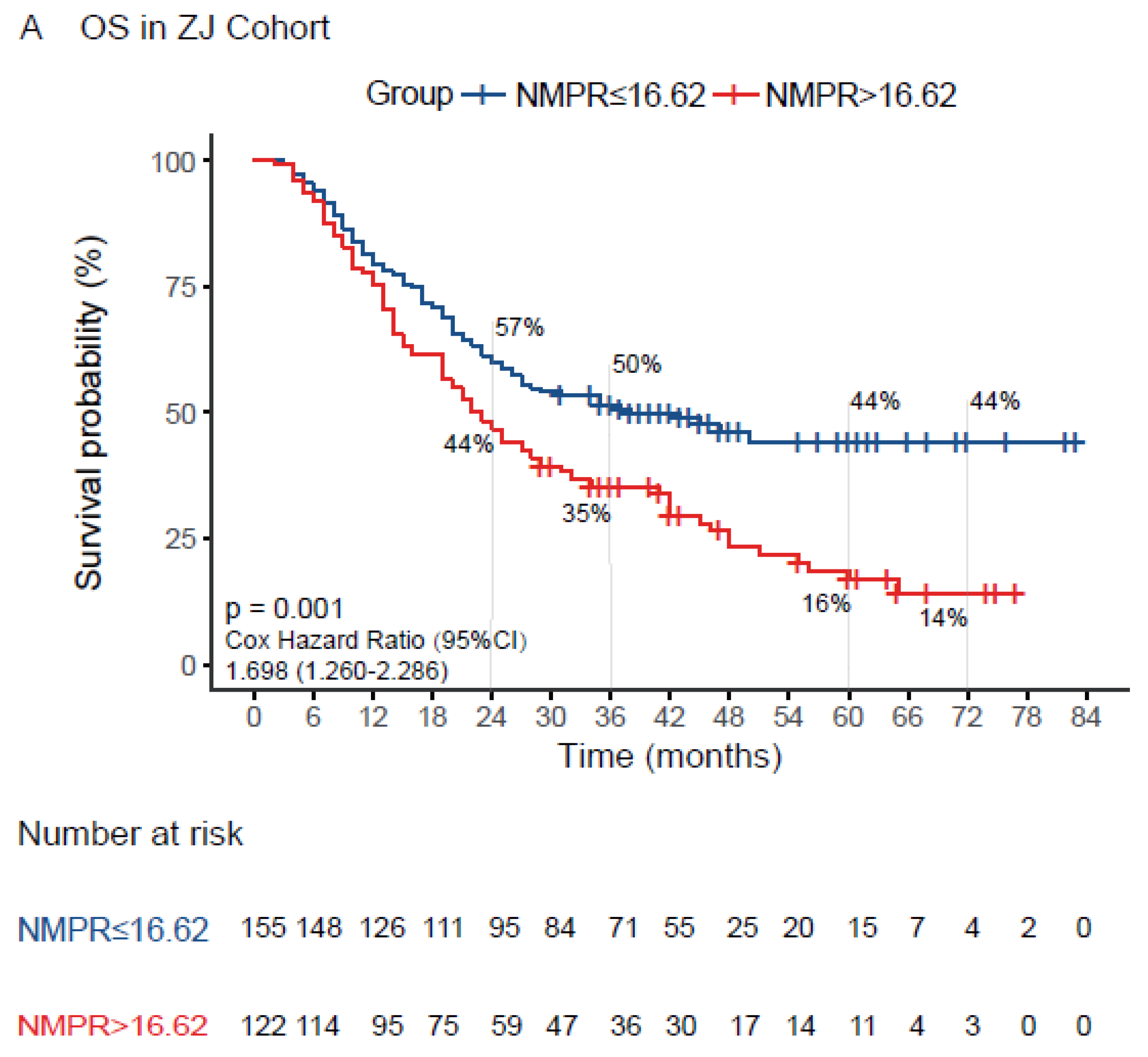
| NMPR (≤16.62/>16.62) | 1.698 | 1.260–2.286 | 0.001 | 1.380 | 1.014–1.878 | 0.040 |
| Variable | Univariate | p-Value | Multivariate | p-Value | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age (≤60 y/>60 y) | 1.869 | 0.657–5.311 | 0.241 | |||
| Gender (Female/Male) | 2.591 | 0.344–19.545 | 0.356 | |||
| Tumor length (≤3 cm/>3 cm) | 3.362 | 1.092–10.345 | 0.034 | 1.148 | 0.303–4.350 | 0.840 |
| Tumor location (Upper + Middle/Lower) | 2.313 | 0.528–10.125 | 0.266 | |||
| Vessel invasion (Negative/Positive) | 2.417 | 0.894–6.537 | 0.082 | |||
| Differentiation (Well + Moderate/Poor) | 1.009 | 0.329–3.097 | 0.987 | |||
| T stage (T1-2/T3-4) | 6.490 | 1.477–28.510 | 0.013 | 3.226 | 0.588–18.141 | 0.176 |
| N stage (N0/N+) | 3.141 | 1.211–8.151 | 0.019 | 2.165 | 0.785–5.972 | 0.136 |
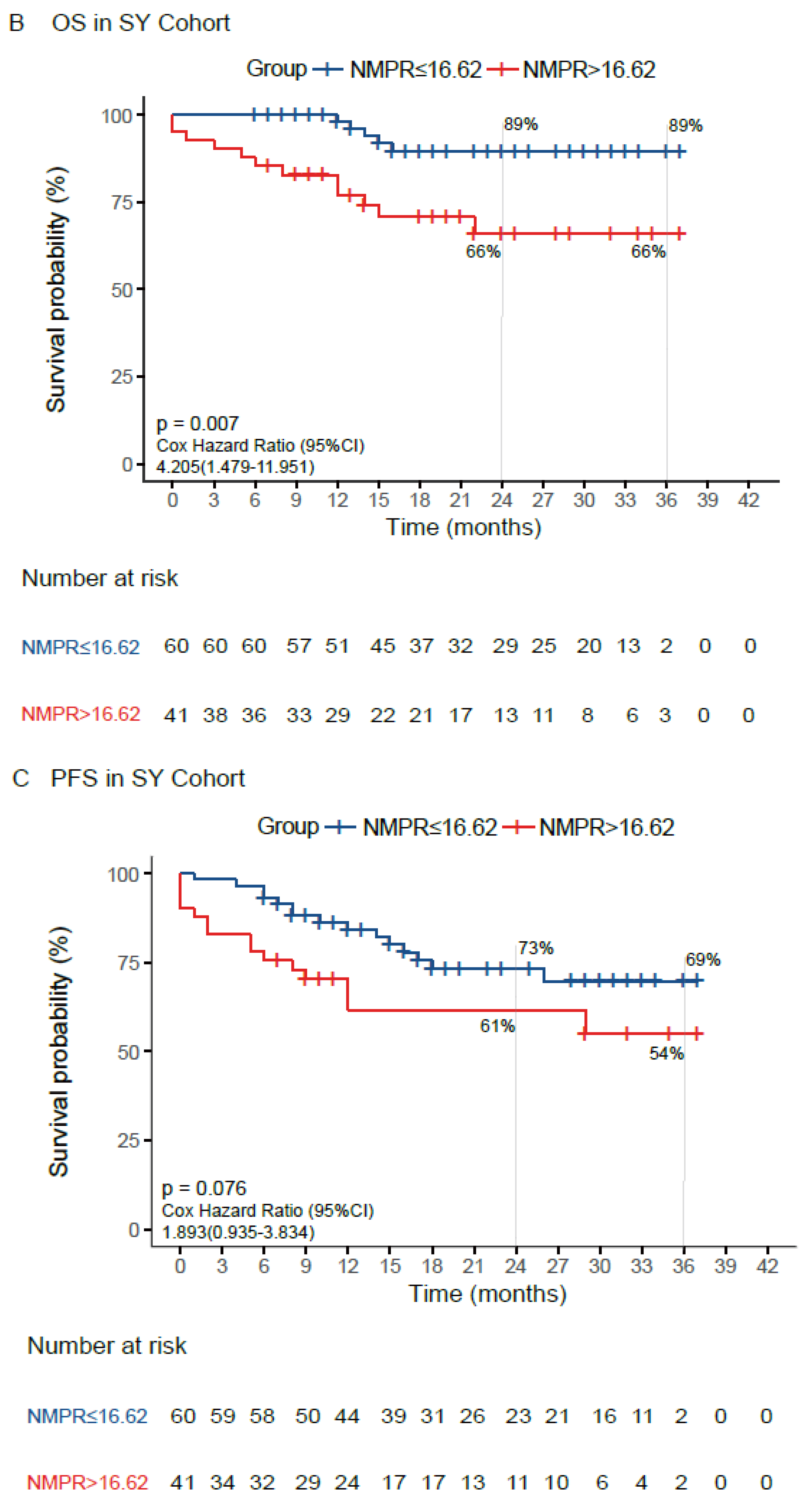
| NMPR (≤16.62/>16.62) | 4.205 | 1.479–11.951 | 0.007 | 3.029 | 1.007–9.112 | 0.049 |
| Variable | Univariate | p-Value | Multivariate | p-Value | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age (≤60 y/>60 y) | 1.701 | 0.800–3.616 | 0.168 | |||
| Gender (Female/Male) | 5.510 | 0.751–40.433 | 0.093 | |||
| Tumor length (≤3 cm/>3 cm) | 2.748 | 1.254–6.019 | 0.012 | 1.406 | 0.592–3.337 | 0.440 |
| Tumor location (Upper + Middle/Lower) | 1.048 | 0.451–2.434 | 0.913 | |||
| Vessel invasion (Negative/Positive) | 4.689 | 2.094–10.499 | <0.001 | 2.788 | 1.134–6.852 | 0.025 |
| Differentiation (Well + Moderate/Poor) | 1.738 | 0.814–3.709 | 0.153 | |||
| T stage (T1-2/T3-4) | 5.164 | 1.959–13.612 | 0.001 | 2.709 | 0.915–8.023 | 0.072 |
| N stage (N0/N+) | 3.775 | 1.851–7.702 | <0.001 | 1.637 | 0.915–8.023 | 0.224 |
| NMPR (≤16.62/>16.62) | 1.893 | 0.935–3.834 | 0.076 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, M.-Y.; Zuo, Z.-G.; Cao, F.-J.; Yu, Y.-D.; Cai, X.-J.; Wan, G.-X. Elevated Preoperative NMPR Predicts an Unfavorable Chance of Survival in Resectable Esophageal Squamous Cell Carcinoma. Medicina 2022, 58, 1808. https://doi.org/10.3390/medicina58121808
Peng M-Y, Zuo Z-G, Cao F-J, Yu Y-D, Cai X-J, Wan G-X. Elevated Preoperative NMPR Predicts an Unfavorable Chance of Survival in Resectable Esophageal Squamous Cell Carcinoma. Medicina. 2022; 58(12):1808. https://doi.org/10.3390/medicina58121808
Chicago/Turabian StylePeng, Meng-Ying, Zhi-Gang Zuo, Feng-Jun Cao, Yuan-Dong Yu, Xiao-Jun Cai, and Guo-Xing Wan. 2022. "Elevated Preoperative NMPR Predicts an Unfavorable Chance of Survival in Resectable Esophageal Squamous Cell Carcinoma" Medicina 58, no. 12: 1808. https://doi.org/10.3390/medicina58121808
APA StylePeng, M.-Y., Zuo, Z.-G., Cao, F.-J., Yu, Y.-D., Cai, X.-J., & Wan, G.-X. (2022). Elevated Preoperative NMPR Predicts an Unfavorable Chance of Survival in Resectable Esophageal Squamous Cell Carcinoma. Medicina, 58(12), 1808. https://doi.org/10.3390/medicina58121808






