Large Intrathoracic Desmoid Tumor and Re-Expansion Pulmonary Edema: Case Report and Review of the Literature
Abstract
1. Introduction
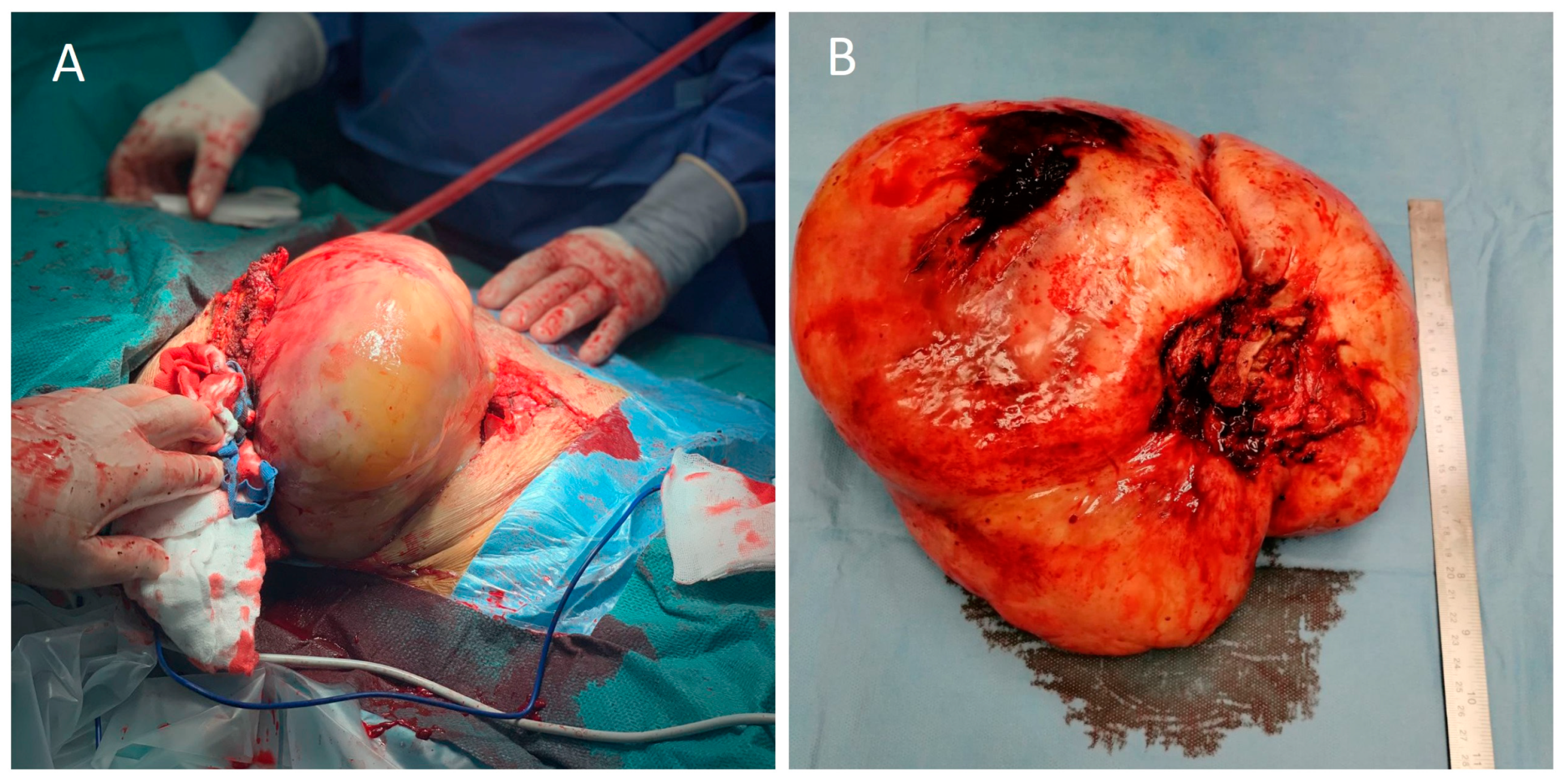
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Wu, C.; Amini-Nik, S.; Nik-Amini, S.; Nadesan, P.; Stanford, W.L.; Alman, B.A. Aggressive Fibromatosis (Desmoid Tumor) Is Derived from Mesenchymal Progenitor Cells. Cancer Res. 2010, 70, 7690–7698. [Google Scholar] [CrossRef]
- Xu, H.; Koo, H.J.; Lim, S.; Lee, J.W.; Lee, H.N.; Kim, D.K.; Song, J.S.; Kim, M.Y. Desmoid-Type Fibromatosis of the Thorax: CT, MRI, and FDG PET Characteristics in a Large Series From a Tertiary Referral Center. Medicine 2015, 94, e1547. [Google Scholar] [CrossRef]
- Ganeshan, D.; Amini, B.; Nikolaidis, P.; Assing, M.; Vikram, R. Current Update on Desmoid Fibromatosis. JCAT 2019, 43, 29–38. [Google Scholar] [CrossRef]
- Murat, A.; Arslan, A.; Balcı, A.E. Re-expansion Pulmonary Edema. Acta Radiol. 2004, 45, 431–433. [Google Scholar] [CrossRef]
- Cantey, E.P.; Walter, J.M.; Corbridge, T.; Barsuk, J.H. Complications of Thoracentesis: Incidence, Risk Factors, and Strategies for Prevention. Curr. Opin. Pulm. Med. 2016, 22, 378–385. [Google Scholar] [CrossRef]
- Fang, H.; Xu, L.; Zhu, F.; Xia, Z. Re-Expansion Pulmonary Edema Post-Pneumothorax. Burn. Trauma 2020, 8, tkaa032. [Google Scholar] [CrossRef]
- Alman, B.; Attia, S.; Baumgarten, C.; Benson, C.; Blay, J.-Y.; Bonvalot, S.; Breuing, J.; Cardona, K.; Casali, P.G.; van Coevorden, F.; et al. The Desmoid Tumor Working Group. The Management of Desmoid Tumours: A Joint Global Consensus-Based Guideline Approach for Adult and Paediatric Patients. EJC 2020, 127, 96–107. [Google Scholar] [CrossRef]
- Kabiri, E.; Al Aziz, S.; El Maslout, A.; Benosman, A. Desmoid Tumors of the Chest Wall. Eur. J. Cardiothorac Surg. 2001, 19, 580–583. [Google Scholar] [CrossRef]
- Iqbal, M.; Rossoff, L.J.; Kahn, L.; Lackner, R.P. Intrathoracic Desmoid Tumor Mimicking Primary Lung Neoplasm. Ann. Cardiothorac. Surg. 2001, 71, 1698–1700. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Chen, X.; Cheng, R.; Yang, X.; Chen, H. Re-Expansion Pulmonary Edema after Resection of Cerebellar Lesion in a Patient with Bronchial Occupying Lesion. Medicine 2019, 98, e15046. [Google Scholar] [CrossRef]
- Havránková, E.; Šteňová, E.; Olejárová, I. Re-Expansion Pulmonary Oedema-Fatal Complication of Mediastinal Tumour Removal. Cor. Vasa 2013, 55, e533–e535. [Google Scholar] [CrossRef]
- Yanagidate, F.; Dohi, S.; Hamaya, Y.; Tsujito, T. Reexpansion Pulmonary Edema after Thoracoscopic Mediastinal Tumor Resection. Anesth. Analg. 2001, 92, 1416–1417. [Google Scholar] [CrossRef]
- Matsumiya, N.; Dohi, S.; Kimura, T.; Naito, H. Reexpansion Pulmonary Edema after Mediastinal Tumor Removal. Anesth. Analg. 1991, 73, 646–648. [Google Scholar] [CrossRef]
- Sagara, Y.; Ohtsuka, T.; Hayashi, K.; Shiraishi, Y.; Komatsu, H.; Katayama, T. Reexpansion Pulmonary Edema after Removal of a Huge Localized Mesothelioma. Nihon Kyobu Geka Gakkai 1993, 41, 1378–1382. [Google Scholar]
- Otomo, A.; Kawatani, M.; Morikawa, T.; Nakazawa, K.; Makita, K. Reexpansion Pulmonary Edema after Removal of a Giant Thoracic Tumor Associated with Long-Time Lung Collapse and Mediastinal Shift. Jpn. J. Anesthesiol. 2004, 53, 291–293. [Google Scholar]
- Mihara, T.; Kurahashi, K. Re-Expansion Pulmonary Edema (RPE) during Surgery for Intraabdominal Giant Tumor. Jpn. J. Anesthesiol. 2008, 57, 191–196. [Google Scholar]
- Hari, J.; Arai, M.; Kosaka, Y.; Toda, M.; Kuroiwa, M.; Okamoto, H. Case Report of Re-Expansion Pulmonary Edema in a Patient with Anorexia Nervosa after Removal of a Huge Ovarian Tumor. Jpn. J. Anesthesiol. 2014, 63, 435–438. [Google Scholar]
- Sherman, S.C. Reexpansion Pulmonary Edema: A Case Report and Review of the Current Literature. J. Emerg. Med. 2003, 24, 23–27. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Shimizu, F.; Shimizu, S.; Urasawa, M.; Tanaka, S.; Kawamata, M. Severe Re-Expansion Pulmonary Edema Induced by One-Lung Ventilation. Respir. Care 2015, 60, e134–e140. [Google Scholar] [CrossRef]
- Sohara, Y. Reexpansion Pulmonary Edema. Ann. Thorac. Cardiovasc. Surg. 2008, 14, 205–209. [Google Scholar]
- Mokotedi, C.M.; Balik, M. Is the Mechanism of Re-Expansion Pulmonary Oedema in a Heart–Lung Interaction? BMJ Case Rep. 2017, 2017, bcr2017219340. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pistioli, E.; Soulioti, E.; Kapetanakis, E.I.; Michos, T.P.; Tomos, P.I.; Sidiropoulou, T. Large Intrathoracic Desmoid Tumor and Re-Expansion Pulmonary Edema: Case Report and Review of the Literature. Medicina 2022, 58, 1857. https://doi.org/10.3390/medicina58121857
Pistioli E, Soulioti E, Kapetanakis EI, Michos TP, Tomos PI, Sidiropoulou T. Large Intrathoracic Desmoid Tumor and Re-Expansion Pulmonary Edema: Case Report and Review of the Literature. Medicina. 2022; 58(12):1857. https://doi.org/10.3390/medicina58121857
Chicago/Turabian StylePistioli, Efstathia, Eleftheria Soulioti, Emmanouil I. Kapetanakis, Thrasyvoulos P. Michos, Periklis I. Tomos, and Tatiana Sidiropoulou. 2022. "Large Intrathoracic Desmoid Tumor and Re-Expansion Pulmonary Edema: Case Report and Review of the Literature" Medicina 58, no. 12: 1857. https://doi.org/10.3390/medicina58121857
APA StylePistioli, E., Soulioti, E., Kapetanakis, E. I., Michos, T. P., Tomos, P. I., & Sidiropoulou, T. (2022). Large Intrathoracic Desmoid Tumor and Re-Expansion Pulmonary Edema: Case Report and Review of the Literature. Medicina, 58(12), 1857. https://doi.org/10.3390/medicina58121857





