In-Hospital and Long-Term Outcomes in Patients with Head and Neck Cancer Bleeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
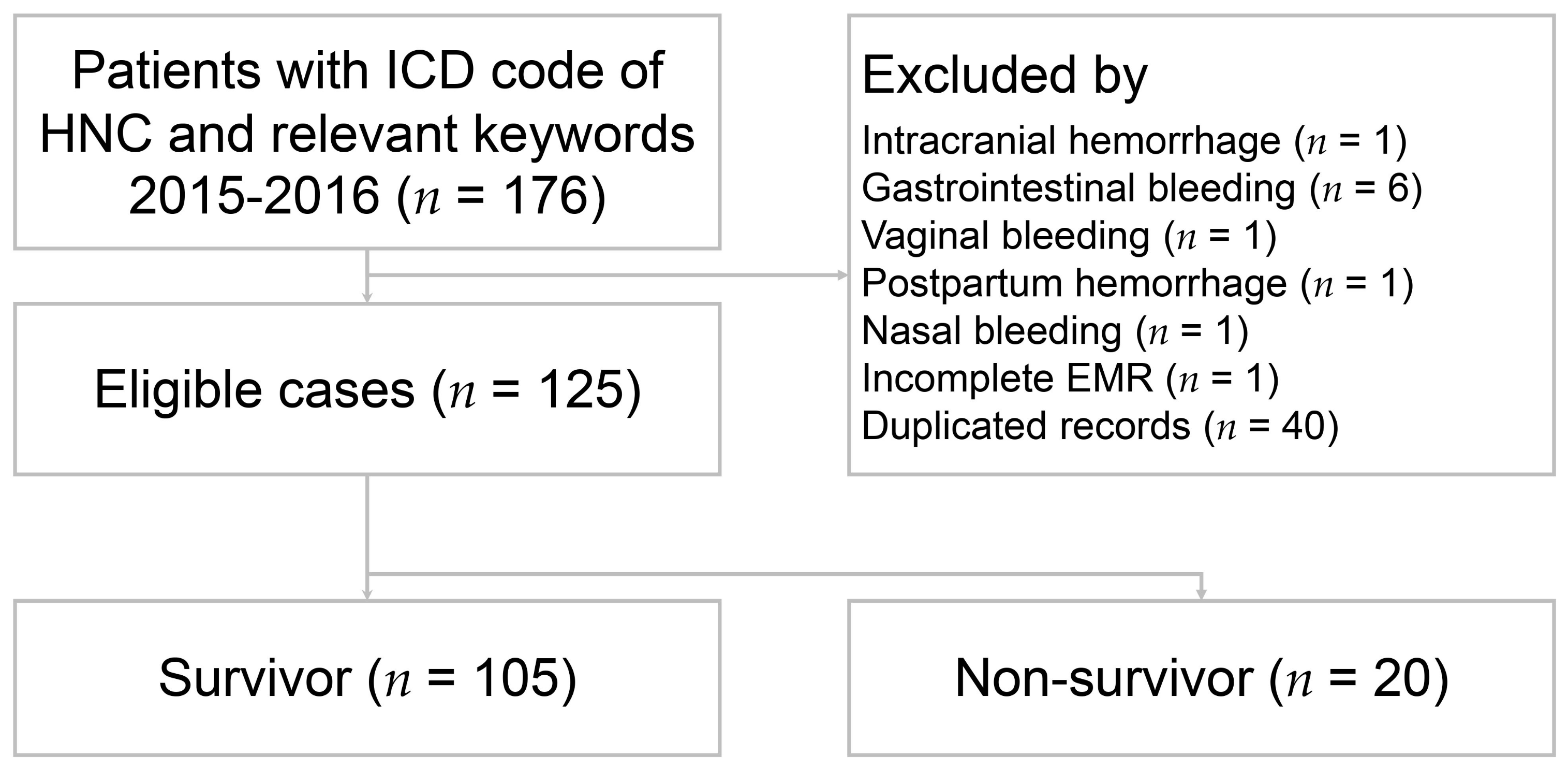
2.2. Patient Selection and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics between Survivors and Non-Survivors
3.2. Bleeding and Various Treatment Modalities
3.3. Univariate and Multivariate Logistic Regression Analyses of in-Hospital Mortality
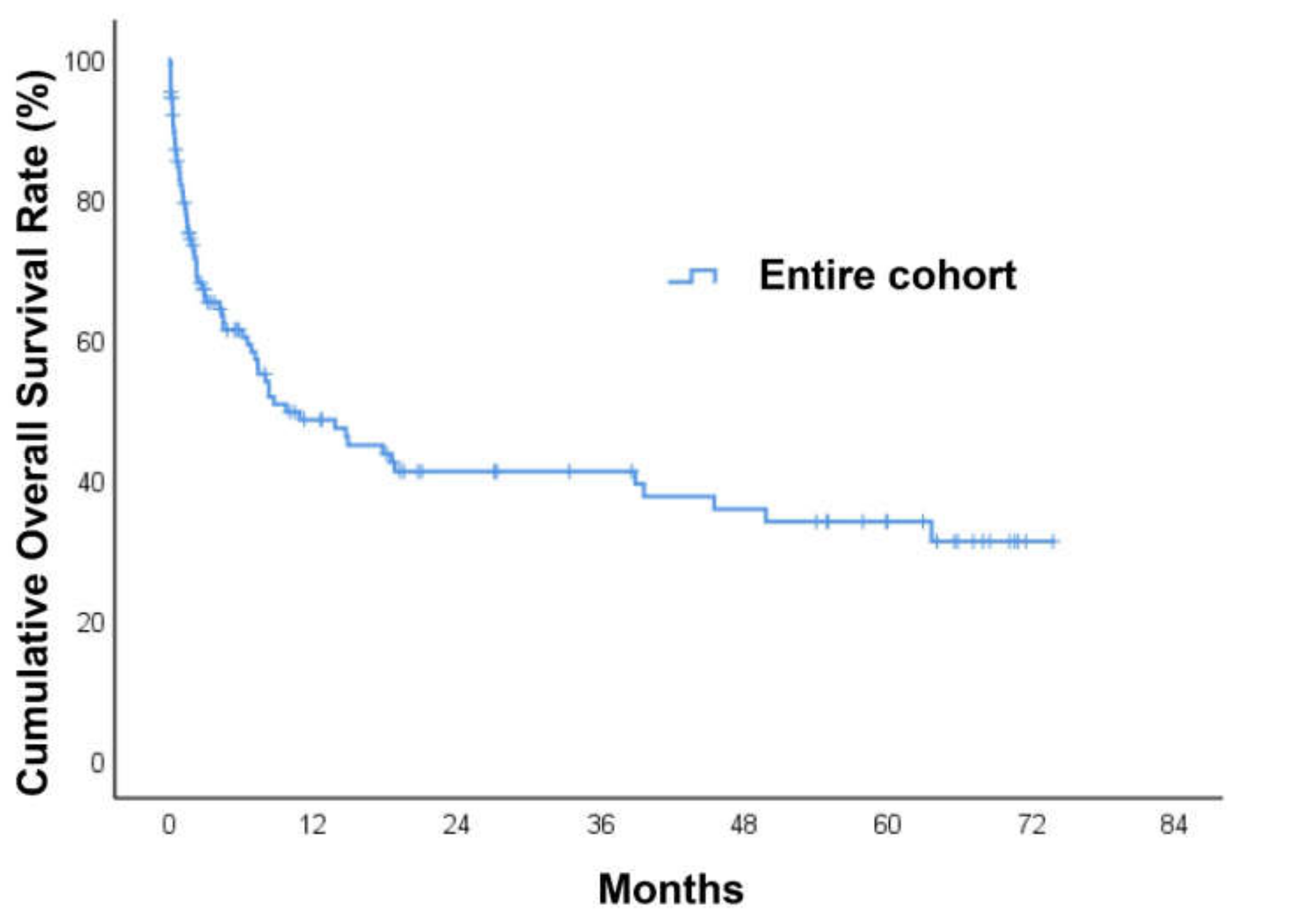
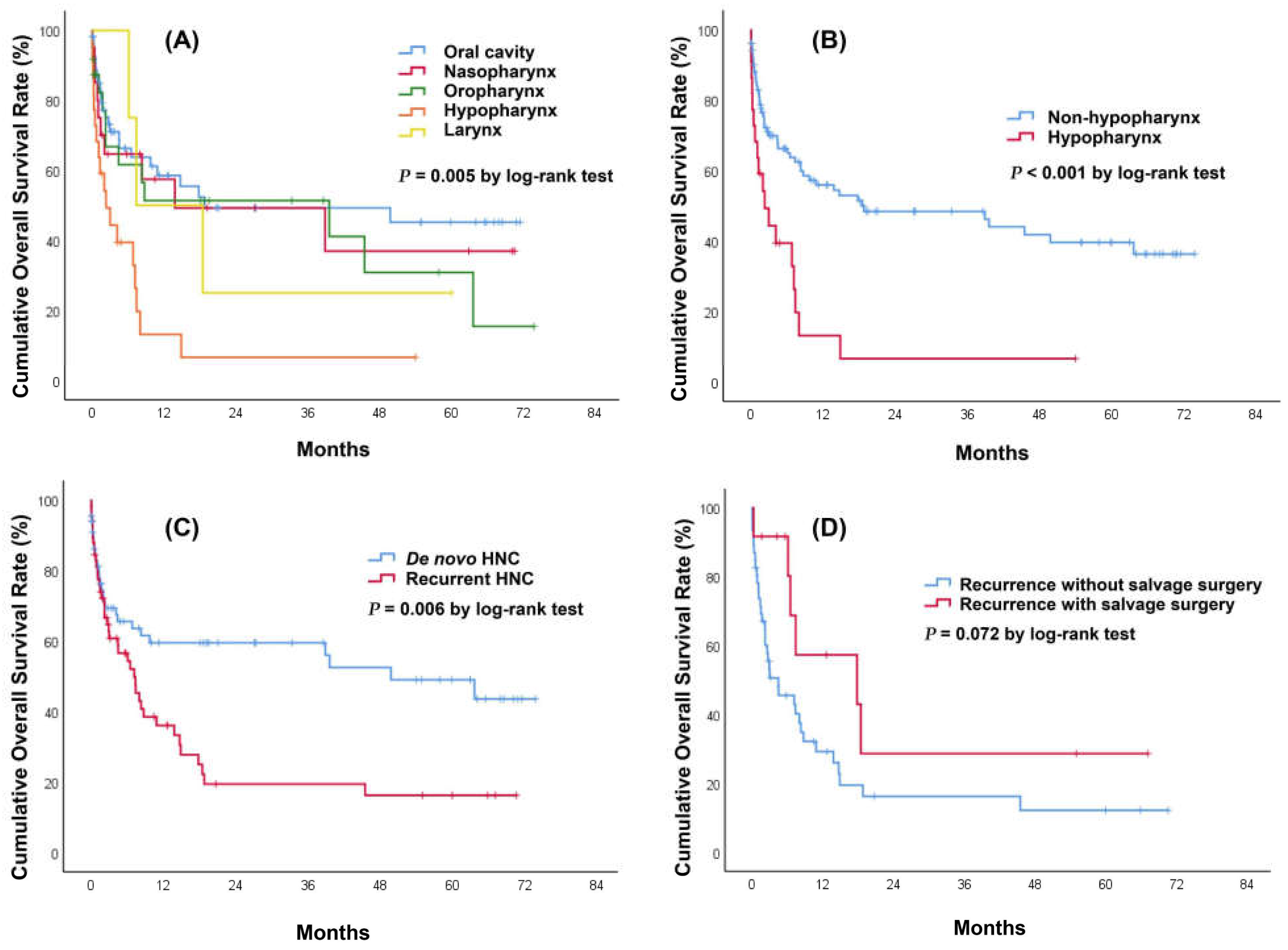
3.4. Long-Term Mortality and Survival Analysis for Patients with Head and Neck Cancer (HNC) Bleeding
3.5. Recurrent Bleeding Assessment in HNC Patients
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yasumatsu, R.; Wakasaki, T.; Hashimoto, K.; Nakashima, K.; Manako, T.; Taura, M.; Matsuo, M.; Nakagawa, T. Monitoring the neutrophil-to-lymphocyte ratio may be useful for predicting the anticancer effect of nivolumab in recurrent or metastatic head and neck cancer. Head Neck 2019, 41, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Gosho, M.; Adachi, M.; Ii, R.; Matsumoto, S.; Miyamoto, H.; Hirose, Y.; Nishimura, B.; Tanaka, S.; Wada, T.; et al. The Geriatric Nutritional Risk Index as a Prognostic Factor in Patients with Advanced Head and Neck Cancer. Laryngoscope 2021, 131, E151–E156. [Google Scholar] [CrossRef] [PubMed]
- Bergamini, C.; Ferris, R.L.; Xie, J.; Mariani, G.; Ali, M.; Holmes, W.C.; Harrington, K.; Psyrri, A.; Cavalieri, S.; Licitra, L. Bleeding complications in patients with squamous cell carcinoma of the head and neck. Head Neck 2021, 43, 2844–2858. [Google Scholar] [CrossRef]
- Chou, C.-P.; Lai, W.-A.; Pan, B.-L.; Yang, Y.-H.; Huang, K.-S. Effects of Hospice Care for Terminal Head and Neck Cancer Patients: A Nationwide Population-Based Matched Cohort Study. J. Palliat. Med. 2021, 24, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-L.; Yu, K.J.; Chiang, C.-J.; Chen, T.-C.; Wang, C.-P. Head and neck cancer incidence trends in Taiwan, 1980~2014. Int. J. Head Neck Sci. 2017, 1, 180–189. [Google Scholar]
- Boas, P.P.V.; De Castro-Afonso, L.H.; Monsignore, L.M.; Nakiri, G.S.; De Mello-Filho, F.V.; Abud, D.G. Endovascular Management of Patients with Head and Neck Cancers Presenting with Acute Hemorrhage: A Single-Center Retrospective Study. Cardiovasc. Interv. Radiol. 2016, 40, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.; Burns, J.; Farinhas, J.; Pasquale, D.; Haboosheh, A.; Bello, J.A.; Brook, A. Covered stents safely utilized to prevent catastrophic hemorrhage in patients with advanced head and neck malignancy. J. Neurointerv. Surg. 2012, 4, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Gemmete, J.; Chaudhary, N.; Pandey, A.; Ansari, S. Acute Life-Threatening Hemorrhage in Patients with Head and Neck Cancer Presenting with Carotid Blowout Syndrome: Follow-Up Results after Initial Hemostasis with Covered-Stent Placement. Am. J. Neuroradiol. 2011, 32, 743–747. [Google Scholar] [CrossRef]
- Jacobi, C.; Gahleitner, C.; Bier, H.; Knopf, A. Chemoradiation and local recurrence of head and neck squamous cell carcinoma and the risk of carotid artery blowout. Head Neck 2019, 41, 3073–3079. [Google Scholar] [CrossRef]
- Liang, N.L.; Guedes, B.D.; Duvvuri, U.; Singh, M.J.; Chaer, R.A.; Makaroun, M.S.; Sachdev, U. Outcomes of interventions for carotid blowout syndrome in patients with head and neck cancer. J. Vasc. Surg. 2016, 63, 1525–1530. [Google Scholar] [CrossRef]
- Lu, H.J.; Chen, K.-W.; Chen, M.-H.; Chu, P.-Y.; Tai, S.-K.; Wang, L.-W.; Chang, P.M.-H.; Yang, M.-H. Predisposing factors, management, and prognostic evaluation of acute carotid blowout syndrome. J. Vasc. Surg. 2013, 58, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Roh, J.-L.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Risk factors for competing non-cancer mortality after definitive treatment for advanced-stage head and neck cancer. Oral Dis. 2018, 24, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Gama, R.R.; Song, Y.; Zhang, Q.; Brown, M.C.; Wang, J.; Habbous, S.; Tong, L.; Huang, S.H.; O’Sullivan, B.; Waldron, J.; et al. Body mass index and prognosis in patients with head and neck cancer. Head Neck 2017, 39, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.G.; Noble, S.I. Management of Terminal Hemorrhage in Patients with Advanced Cancer: A Systematic Literature Review. J. Pain Symptom Manag. 2009, 38, 913–927. [Google Scholar] [CrossRef]
- Wong, D.J.Y.; Donaldson, C.; Lai, L.T.; Coleman, A.; Giddings, C.; Slater, L.-A.; Chandra, R. Safety and effectiveness of endovascular embolization or stent-graft reconstruction for treatment of acute carotid blowout syndrome in patients with head and neck cancer: Case series and systematic review of observational studies. Head Neck 2018, 40, 846–854. [Google Scholar] [CrossRef]
- Gebhardt, B.J.; Vargo, J.A.; Ling, D.; Jones, B.; Mohney, M.; Clump, D.A.; Ohr, J.P.; Ferris, R.L.; Heron, D.E. Carotid Dosimetry and the Risk of Carotid Blowout Syndrome After Reirradiation With Head and Neck Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. 2018, 101, 195–200. [Google Scholar] [CrossRef]
- Lee, C.-W.; Yang, C.-Y.; Chen, Y.-F.; Huang, A.; Wang, Y.-H.; Liu, H.-M. CT Angiography Findings in Carotid Blowout Syndrome and Its Role as a Predictor of 1-Year Survival. Am. J. Neuroradiol. 2013, 35, 562–567. [Google Scholar] [CrossRef]
- Ahn, S.H.; Haas, R.A. Interventional Management of Head and Neck Emergencies: Carotid Blowout. Semin. Interv. Radiol. 2013, 30, 245–248. [Google Scholar] [CrossRef][Green Version]
- Krol, E.; Brandt, C.T.; Blakeslee-Carter, J.; Ahanchi, S.S.; Dexter, D.J.; Karakla, D.; Panneton, J.M. Vascular interventions in head and neck cancer patients as a marker of poor survival. J. Vasc. Surg. 2019, 69, 181–189. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Wang, C.-P.; Wang, C.-C.; Jiang, R.-S.; Lin, J.-C.; Liu, S.-A. Carotid blowout in patients with head and neck cancer: Associated factors and treatment outcomes. Head Neck 2015, 37, 265–272. [Google Scholar] [CrossRef]
- Chang, F.-C.; Lirng, J.-F.; Luo, C.-B.; Wang, S.-J.; Wu, H.-M.; Guo, W.-Y.; Teng, M.M.H.; Chang, C.-Y. Patients with head and neck cancers and associated postirradiated carotid blowout syndrome: Endovascular therapeutic methods and outcomes. J. Vasc. Surg. 2008, 47, 936–945. [Google Scholar] [CrossRef]
- Cannavale, A.; Corona, M.; Nardis, P.; De Rubeis, G.; Cannavale, G.; Santoni, M.; De Gyurgyokai, S.Z.; Catalano, C.; Bezzi, M. Computed Tomography Angiography findings can predict massive bleeding in head and neck tumours. Eur. J. Radiol. 2020, 125, 108910. [Google Scholar] [CrossRef]
- Heller, K.S.; Strong, E.W. Carotid arterial hemorrhage after radical head and neck surgery. Am. J. Surg. 1979, 138, 607–610. [Google Scholar] [CrossRef]
- Upile, T.; Triaridis, S.; Kirkland, P.; Archer, D.; Searle, A.; Irving, C.; Evans, P.R. The management of carotid artery rupture. Eur. Arch. Oto-Rhino-Laryngol. 2005, 262, 555–560. [Google Scholar] [CrossRef]
- Sanders, O.; Pathak, S. Hypopharyngeal Cancer. In StatPearl; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Arends, C.R.; Petersen, J.F.; Van Der Noort, V.; Timmermans, A.J.; Leemans, C.R.; De Bree, R.; Brekel, M.W.V.D.; Stuiver, M.M. Optimizing Survival Predictions of Hypopharynx Cancer: Development of a Clinical Prediction Model. Laryngoscope 2019, 130, 2166–2172. [Google Scholar] [CrossRef]
- Wiegand, S.; Zimmermann, A.; Wilhelm, T.; Werner, J.A. Survival after Distant Metastasis in Head and Neck Cancer. Anticancer Res. 2015, 35, 5499–5502. [Google Scholar]
- Cannon, J.W. Hemorrhagic Shock. N. Engl. J. Med. 2018, 378, 370–379. [Google Scholar] [CrossRef]
- Song, J.; Cho, H.; Park, D.; Moon, S.; Kim, J.; Ahn, S.; Lee, S.-G.; Park, J. Vasoactive–Inotropic Score as an Early Predictor of Mortality in Adult Patients with Sepsis. J. Clin. Med. 2021, 10, 495. [Google Scholar] [CrossRef]
- Bonanno, F.G. The need for a physiological classification of hemorrhagic shock. J. Emerg. Trauma Shock 2020, 13, 177–182. [Google Scholar] [CrossRef]
- Rowe, K.; Fletcher, S.J. The emergency management of the patient presenting with shock. Acute Med. 2011, 10, 103–105. [Google Scholar] [CrossRef]

| Variable | Survivors N = 105 | Nonsurvivors N = 20 | p Value |
|---|---|---|---|
| Age (year) | 11.1 | 11.1 | 0.650 |
| Male | 97 (92.4) | 17 (85.0) | 0.381 |
| Systolic blood pressure (mmHg) | 34.3 | 40.8 | 0.917 |
| Diastolic blood pressure (mmHg) | 17.7 | 19.8 | 0.457 |
| Heart rate > 110 (beats/min) | 32 (30.5) | 9 (47.4) | 0.150 |
| Smoking history | 79 (75.2) | 18 (90.0) | 0.240 |
| Betel nut chewer | 69 (65.7) | 12 (60.0) | 0.624 |
| Previous medical history | |||
| Hypertension | 30 (28.6) | 8 (40.0) | 0.309 |
| Diabetes mellitus | 18 (17.1) | 6 (30.0) | 0.216 |
| Coronary artery disease | 5 (4.8) | 1 (5.0) | 1.000 |
| Chronic kidney disease | 5 (4.7) | 1 (5.0) | 1.000 |
| Other malignancy | 6 (5.7) | 2 (10.0) | 0.613 |
| Prior stroke | 9 (8.6) | 0 (0) | 0.352 |
| Liver cirrhosis | 10 (9.5) | 1 (5.0) | 1.000 |
| Laboratory exam | |||
| White cell count (103/uL) n = 123 | 7.9 | 5.9 | 0.784 |
| Hemoglobin (g/dL) n = 124 | 2.2 | 1.8 | 0.189 |
| Platelet count (103/uL) n = 123 | 102 | 159 | 0.322 |
| INR n = 115 | 0.10 | 0.35 | 0.048 |
| Creatinine (mg/dL) n = 123 | 0.94 | 1.84 | 0.278 |
| ALT (U/L) n = 102 | 18.1 | 142 | 0.111 |
| Variable | Survivors N = 105 | Non-Survivors N = 20 | p Value |
|---|---|---|---|
| Cancer site | 0.105 | ||
| Oral cavity | 48 (45.7) | 7 (35.0) | |
| Nasopharynx | 17 (16.2) | 3 (15.0) | |
| Oropharynx | 22 (21.0) | 2 (10.0) | |
| Hypopharynx | 14 (13.3) | 8 (40.0) | |
| Larynx | 4 (3.8) | 0 (0) | |
| T stage | 0.294 | ||
| T ≤ 2 | 33 (31.4) | 3 (15.0) | |
| T > 2 | 68 (64.8) | 16 (80.0) | |
| Unknown | 4 (3.8) | 1 (5.0) | |
| N stage | 0.656 | ||
| N0 | 34 (32.4) | 6 (30.0) | |
| N+ | 64 (61.0) | 14 (70.0) | |
| Unknown | 7 (6.7) | 0 (0) | |
| M stage | 0.157 | ||
| M0 | 95 (90.5) | 16 (80.0) | |
| M1 | 4 (3.8) | 3 (15.0) | |
| Unknown | 6 (5.7) | 1 (5.0) | |
| Pathology type | 0.745 | ||
| Squamous cell carcinoma | |||
| Keratinizing carcinoma | 96 (91.4) | 18 (90.0) | |
| Non-keratinizing carcinoma | 7 (6.7) | 2 (10.0) | |
| Sarcomatoid carcinoma | 1 (1.0) | 0 (0) | |
| Adenocarcinoma | 1 (1.0) | 0 (0) | |
| Initial cancer treatment | |||
| Surgical resection | 51 (48.6) | 8 (40.0) | 0.482 |
| Chemoradiation | 83 (79.0) | 17 (85.0) | 0.762 |
| Neck dissection | 45 (42.9) | 7 (35.0) | 0.514 |
| Flap reconstruction | 36 (34.3) | 7 (35.0) | 0.951 |
| Local recurrence | 49 (46.7) | 9 (45.0) | 0.891 |
| Bleeding cause | 0.268 | ||
| Tumor related | 78 (74.3) | 18 (90.0) | |
| Pseudoaneurysm | 19 (18.1) | 2 (10.0) | |
| Post-operative complication | 8 (7.6) | 0 (0) | |
| Bleeding type | 0.120 | ||
| Self-limited | 40 (38.1) | 4 (20.0) | |
| Active bleeding | 65 (61.9) | 16 (80.0) | |
| Emergent CTA | 49 (46.7) | 13 (65.0) | 0.133 |
| Bleeding treatment | 0.952 | ||
| Supportive care | 77 (73.3) | 16 (80.0) | |
| Embolization | 19 (18.1) | 4 (20) | |
| Covered stent | 5 (4.8) | 0 (0) | |
| Surgical ligation | 3 (2.9) | 0 (0) | |
| Primary repair | 1 (1.0) | 0 (0) | |
| Inotropic support | 3 (2.9) | 4 (20.0) | 0.013 |
| Blood transfusion | 54 (51.4) | 11 (55.0) | 0.770 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95%CI) | p Value | OR (95%CI) | p Value | |
| Age | 0.99 (0.95, 1.03) | 0.647 | ||
| Male | 0.47 (0.11, 1.94) | 0.295 | ||
| Heart rate > 110 (beats/min) | 2.05 (0.76, 5.53) | 0.155 | ||
| Inotropic support | 8.50 (1.74, 41.56) | 0.008 | 10.41 (1.81, 59.84) | 0.009 * |
| Hypertension | 1.67 (0.62, 4.48) | 0.312 | ||
| Diabetes mellitus | 2.07 (0.70, 6.12) | 0.187 | ||
| Hypopharyngeal cancer | 4.33 (1.51, 12.47) | 0.007 | 4.32 (1.29, 14.46) | 0.018 * |
| Surgical resection | 0.71 (0.27, 1.87) | 0.483 | ||
| Chemoradiation | 1.50 (0.40, 5.59) | 0.544 | ||
| Neck dissection | 0.72 (0.27, 1.95) | 0.515 | ||
| Flap reconstruction | 1.03 (0.38, 2.82) | 0.951 | ||
| T stage | 1.84 (1.01, 3.37) | 0.050 | 1.36 (0.70, 2.64) | 0.368 |
| N stage | 1.05 (0.66, 1.68) | 0.827 | ||
| M stage | 4.45 (0.91, 21.79) | 0.065 | 5.90 (1.07, 32.70) | 0.042 * |
| Local recurrence | 0.94 (0.36, 2.44) | 0.891 | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Age | 0.99 (0.97, 1.01) | 0.225 | ||
| Male | 0.63 (0.30, 1.31) | 0.215 | ||
| Heart rate > 110 (beats/min) | 1.95 (1.18, 3.23) | 0.010 | 2.02 (1.16, 3.51) | 0.013 * |
| Inotropic support | 5.71 (2.40, 13.57) | <0.001 | 3.25 (1.20, 8.82) | 0.021 * |
| Hypertension | 0.76 (0.44, 1.30) | 0.309 | ||
| Diabetes mellitus | 1.02 (0.56, 1.07) | 0.943 | ||
| Hypopharyngeal cancer | 2.79 (1.60, 4.85) | <0.001 | 2.22 (1.21, 4.06) | 0.010 * |
| Surgical resection | 0.57 (0.35, 0.92) | 0.023 | 0.74 (0.42, 1.31) | 0.301 |
| Chemoradiation | 2.55 (1.21, 5.37) | 0.014 | 2.10 (0.89, 4.94) | 0.094 |
| Neck dissection | 0.76 (0.47, 1.23) | 0.260 | ||
| Flap reconstruction | 0.88 (0.53, 1.45) | 0.603 | ||
| T stage | 1.40 (1.10, 1.78) | 0.006 | 1.26 (0.96, 1.65) | 0.092 |
| N stage | 1.33 (1.06, 1.68) | 0.015 | 1.19 (0.90, 1.58) | 0.213 |
| M stage | 2.13 (0.76, 5.96) | 0.149 | ||
| Local recurrence | 1.95 (1.20, 3.19) | 0.007 | 1.76 (0.99, 3.12) | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yen, C.-C.; Ho, C.-F.; Wu, C.-C.; Tsao, Y.-N.; Chaou, C.-H.; Chen, S.-Y.; Ng, C.-J.; Yeh, H. In-Hospital and Long-Term Outcomes in Patients with Head and Neck Cancer Bleeding. Medicina 2022, 58, 177. https://doi.org/10.3390/medicina58020177
Yen C-C, Ho C-F, Wu C-C, Tsao Y-N, Chaou C-H, Chen S-Y, Ng C-J, Yeh H. In-Hospital and Long-Term Outcomes in Patients with Head and Neck Cancer Bleeding. Medicina. 2022; 58(2):177. https://doi.org/10.3390/medicina58020177
Chicago/Turabian StyleYen, Chieh-Ching, Che-Fang Ho, Chia-Chien Wu, Yu-Ning Tsao, Chung-Hsien Chaou, Shou-Yen Chen, Chip-Jin Ng, and Heng Yeh. 2022. "In-Hospital and Long-Term Outcomes in Patients with Head and Neck Cancer Bleeding" Medicina 58, no. 2: 177. https://doi.org/10.3390/medicina58020177
APA StyleYen, C.-C., Ho, C.-F., Wu, C.-C., Tsao, Y.-N., Chaou, C.-H., Chen, S.-Y., Ng, C.-J., & Yeh, H. (2022). In-Hospital and Long-Term Outcomes in Patients with Head and Neck Cancer Bleeding. Medicina, 58(2), 177. https://doi.org/10.3390/medicina58020177






