The Prevalence of Potential Drug-Drug Interactions in CKD-A Retrospective Observational Study of Cerrahpasa Nephrology Unit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design-Setting
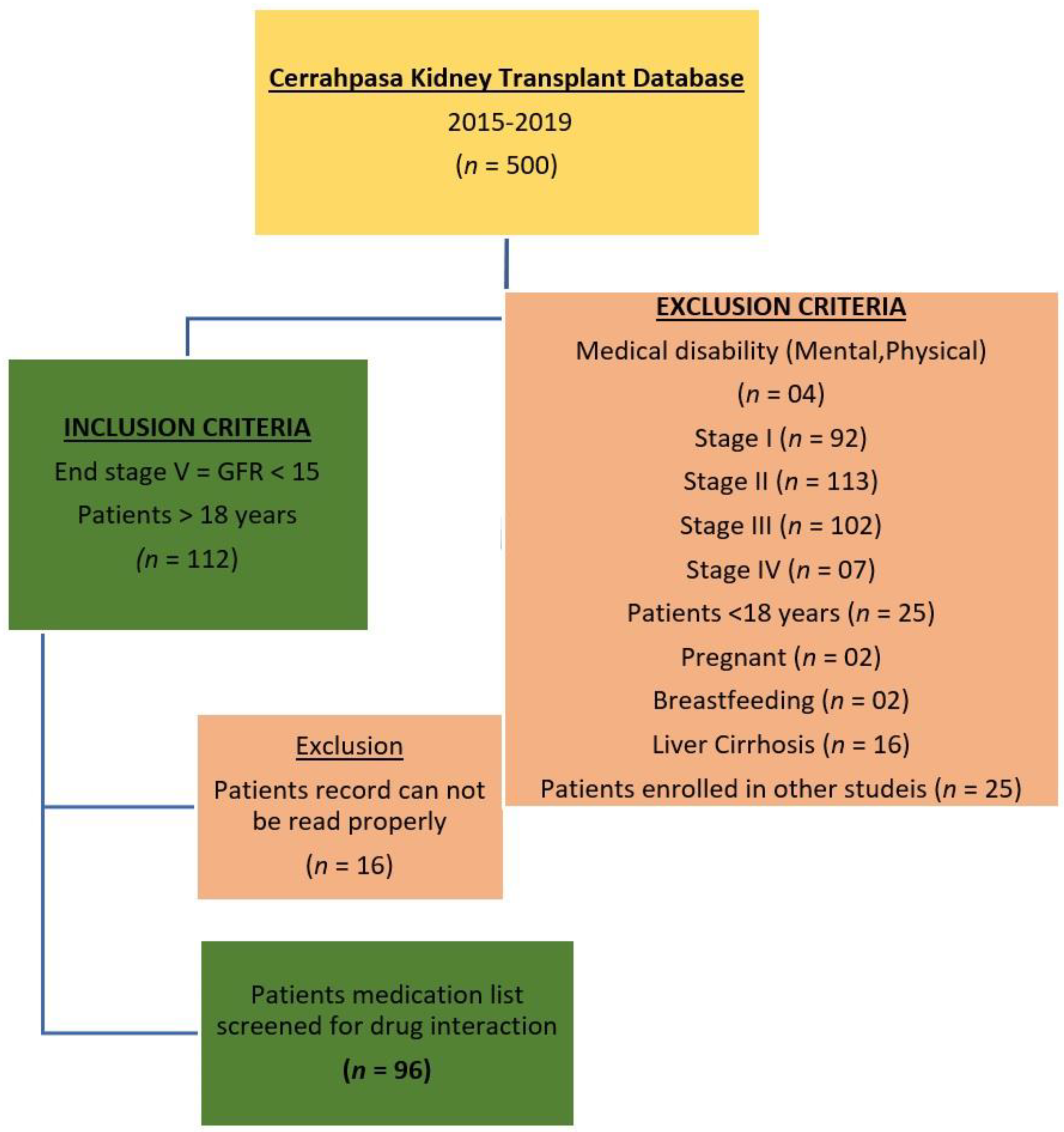
2.2. Data Collection
2.3. Classification of Potential Drug-Drug Interaction
2.4. Evaluation of DDI Frequency
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacGregor, M.S. Chronic kidney disease: Evolving strategies for detection and management of impaired renal function. QJM 2006, 99, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Süleymanlar, G.; Serdengeçti, K.; Altiparmak, M.R.; Jager, K.; Seyahi, N.; Erek, E. Trends in Renal Replacement Therapy in Turkey, 1996–2008. Am. J. Kidney Dis. 2011, 57, 456–465. [Google Scholar] [CrossRef]
- Süleymanlar, G.; Utaş, C.; Arinsoy, T.; Ateş, K.; Altun, B.; Altiparmak, M.R.; Ecder, T.; Yilmaz, M.E.; Çamsari, T.; Başçi, A.; et al. A population-based survey of Chronic REnal Disease In Turkey--the CREDIT study. Nephrol. Dial Transpl. 2011, 26, 1862–1871. [Google Scholar] [CrossRef]
- Stevens, L.A.; Viswanathan, G.; Weiner, D.E. Chronic kidney disease and end-stage renal disease in the elderly population: Current prevalence, future projections, and clinical significance. Adv. Chronic. Kidney Dis. 2010, 17, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Weir, M.R. Hypertension and the Kidney: Perspectives on the Relationship of Kidney Disease and Cardiovascular Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 2045–2050. [Google Scholar] [CrossRef]
- Plantinga, L.C.; Crews, D.C.; Coresh, J.; Miller, E.R., 3rd; Saran, R.; Yee, J.; Hedgeman, E.; Pavkov, M.; Eberhardt, M.S.; Williams, D.E.; et al. Prevalence of chronic kidney disease in US adults with undiagnosed diabetes or prediabetes. Clin. J. Am. Soc. Nephrol. 2010, 5, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Alshamrani, M.; Almalki, A.; Qureshi, M.; Yusuf, O.; Ismail, S. Polypharmacy and Medication-Related Problems in Hemodialysis Patients: A Call for Deprescribing. Pharmacy 2018, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Rama, M.; Viswanathan, G.; Acharya, L.D.; Attur, R.P.; Reddy, P.N.; Raghavan, S.V. Assessment of Drug-Drug Interactions among Renal Failure Patients of Nephrology Ward in a South Indian Tertiary Care Hospital. Indian J. Pharm. Sci. 2012, 74, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 230. [Google Scholar] [CrossRef] [Green Version]
- Lavan, A.H.; Gallagher, P. Predicting risk of adverse drug reactions in older adults. Ther. Adv. Drug Saf. 2016, 7, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.M.; Hajjar, E.R. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin. Geriatr. Med. 2012, 28, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Fattinger, K.; Roos, M.; Vergères, P.; Holenstein, C.; Kind, B.; Masche, U.; Stocker, D.N.; Braunschweig, S.; Kullak-Ublick, G.A.; Galeazzi, R.L.; et al. Epidemiology of drug exposure and adverse drug reactions in two swiss departments of internal medicine. Br. J. Clin. Pharmacol. 2000, 49, 158–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Díaz, G.; Pérez-Pico, A.M.; Suárez-Santisteban, M.Á.; García-Bernalt, V.; Mayordomo, R.; Dorado, P. Prevalence of Potential Drug-Drug Interaction Risk among Chronic Kidney Disease Patients in a Spanish Hospital. Pharmaceutics 2020, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Marquito, A.B.; Fernandes, N.M.; Colugnati, F.A.; de Paula, R.B. Identifying potential drug interactions in chronic kidney disease patients. J. Bras. Nefrol. 2014, 36, 26–34. [Google Scholar] [CrossRef]
- Saleem, A.; Masood, I.; Khan, T.M. Clinical relevancy and determinants of potential drug-drug interactions in chronic kidney disease patients: Results from a retrospective analysis. Integr. Pharm. Res. Pract. 2017, 6, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Al-Ramahi, R.; Raddad, A.R.; Rashed, A.O.; Bsharat, A.; Abu-Ghazaleh, D.; Yasin, E.; Shehab, O. Evaluation of potential drug- drug interactions among Palestinian hemodialysis patients. BMC Nephrol. 2016, 17, 96. [Google Scholar] [CrossRef] [Green Version]
- Adibe, M.O.; Ewelum, P.C.; Amorha, K.C. Evaluation of drug-drug interactions among patients with chronic kidney disease in a South-Eastern Nigeria tertiary hospital: A retrospective study. Pan Afr. Med. J. 2017, 28, 199. [Google Scholar] [CrossRef]
- Olumuyiwa, J.F.; Akinwumi, A.A.; Ademola, O.A.; Oluwole, B.A.; Ibiene, E.O. Prevalence and pattern of potential drug-drug interactions among chronic kidney disease patients in south-western Nigeria. Niger. Postgrad. Med. J. 2017, 24, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Khan, M.U.; Haque, I.; Ivan, R.; Dasari, R.; Revanker, M.; Pravina, A.; Kuriakose, S. Evaluation of potential drug—Drug interactions in general medicine ward of teaching hospital in southern India. J. Clin. Diagn. Res. 2015, 9, FC10–FC13. [Google Scholar] [CrossRef]
- McKillop, G.; Joy, J. Patıents’ experıence and perceptıons of polypharmacy ın chronıc kıdney dısease and ıts ımpact on adherent behavıour. J. Ren. Care 2013, 39, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Mason, N.A. Polypharmacy and medication-related complications in the chronic kidney disease patient. Curr. Opin. Nephrol. Hypertens. 2011, 20, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Papotti, B.; Marchi, C.; Adorni, M.P.; Potì, F. Drug-drug interactions in polypharmacy patients: The impact of renal impairment. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100020. [Google Scholar] [CrossRef] [PubMed]
- Nusair, M.B.; Al-Azzam, S.I.; Arabyat, R.M.; Amawi, H.A.; Alzoubi, K.H.; Rabah, A.A. The prevalence and severity of potential drug-drug interactions among adult polypharmacy patients at outpatient clinics in Jordan. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2020, 28, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Doubova Dubova, S.V.; Reyes-Morales, H.; Torres-Arreola, L.D.P.; Suárez-Ortega, M. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv. Res. 2007, 7, 147. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, M.; Malone, D.C.; Skrepnek, G.H.; Abarca, J.; Armstrong, E.P.; Murphy, J.E.; Grizzle, A.J.; Ko, Y.; Woosley, R.L. Potential drug–drug interactions within Veterans Affairs medical centers. Am. J. Health-Syst. Pharm. 2007, 64, 1500–1505. [Google Scholar] [CrossRef]
- Glintborg, B.; Andersen, S.E.; Dalhoff, K. Drug-drug interactions among recently hospitalised patients–frequent but mostly clinically insignificant. Eur. J. Clin. Pharmacol. 2005, 61, 675–681. [Google Scholar] [CrossRef]
- White, J.R.; Campbell, R.K. Dangerous and common drug ınteractıons ın patıents wıth diabetes mellıtus. Endocrinol. Metab. Clin. N. Am. 2000, 29, 789–801. [Google Scholar] [CrossRef]
- Demir, C.; Dogantekin, A.; Gurel, A.; Aydin, S.; Celiker, H. Is there a relationship between serum vaspin levels and insulin resistance in chronic renal failure? Pak. J. Med. Sci. 2019, 35, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Alomar, M.J. Factors affecting the development of adverse drug reactions (Review article). Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2014, 22, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Diksis, N.; Melaku, T.; Assefa, D.; Tesfaye, A. Potential drug-drug interactions and associated factors among hospitalized cardiac patients at Jimma University Medical Center, Southwest Ethiopia. SAGE Open Med. 2019, 7, 2050312119857353. [Google Scholar] [CrossRef] [PubMed]

| Number of Patients | 96 |
|---|---|
| Male, n (%) | 41 (42.7) |
| Female, n (%) | 55 (57.3) |
| Average age (years) | 53 (26–77) |
| Diagnosis | |
| Hypertension, n (%) | 77 (80.2) |
| Diabetes Mellitus, n (%) | 24 (25.0) |
| Hypertension + Diabetes Mellitus, n (%) | 17 (17.7) |
| Other Diseases | 12 (12.5) |
| Number of indication | Patients, n (%) |
| 1 | 10 (10.4) |
| 2 | 56 (58.3) |
| 3 | 22 (22.9) |
| 4 | 7 (7.3) |
| 5 | 1 (1.0) |
| Average indication | 2.3 |
| Interaction | Number of Patients |
|---|---|
| DDIs | 67 (69.8%) |
| No DDIs | 29 (30.2%) |
| Degree | Number of DDIs |
| Minor | 31 (20.8%) |
| Moderate | 112 (75.2%) |
| Major | 6 (4.0%) |
| Total | 149 |
| Medicine Prescribed | p Value (Kruskal Wallis Test) | Interactions | p Value (Mann Whitney U Test) |
|---|---|---|---|
| Electrolytes | 0.000 * | Iron + Electrolytes | 0.000 * |
| Iron | 0.000 * | Calcium channel blockers + β-blockers | 0.000 * |
| β-Blockers | 0.000 * | Aspirin + Electrolytes | 0.000 * |
| Non-steroidal anti-inflammatory drugs | 0.000 * | β-blockers + NSAIDs | 0.000 * |
| Calcium Channel blockers | 0.000 * | Calcium channel blockers + NSAIDs | 0.000 * |
| Aspirin | 0.000 * | Ascorbic acid + Cyanocobalamin | 0.000 * |
| Calcium | 0.000 * | α-blockers + β-blockers | 0.001 * |
| Lansoprazole | 0.000 * | Antidiabetics + β-blockers | 0.001 * |
| Antidiabetics | 0.010 * | Iron + Lansoprazole | 0.001 * |
| Ascorbic acid | 0.010 * | Lansoprazole + Antacids | 0.001 * |
| Cyanocobalamin | 0.010 * | Angiotensin receptor blockers + NSAIDs | 0.003 * |
| Angiotensin Receptor Blockers | 0.022 * | Verapamil + Calcium | 0.003 * |
| Antacids | 0.022 * | Aspirin + Insulin | 0.011 * |
| Angiotensin converting enzyme inhibitors | 0.107 | Calcium + Hydrochlorothiazide | 0.011 * |
| Sucralfate | 0.022 * | Doxazosin + Nifedipine | 0.011 * |
| α-Blockers | 0.022 * | Sucralfate + Electrolytes | 0.011 * |
| Verapamil | 0.022 * | ACE inhibitors + Furosemide | 0.040 * |
| Furosemide | 0.049 * | Furosemide + Sucralfate | 0.040 * |
| Thiazide | 0.482 | Methyldopa + Iron | 0.040 * |
| Doxazosin | 0.107 | NSAIDs + Thiazide | 0.040 * |
| Hydrochlorothiazide | 0.107 | ACE inhibitors + Angiotensin receptor blockers | 0.148 |
| Insulin | 0.107 | ACE inhibitors + Thiazide | 0.040 * |
| Nifedipine | 0.107 | Amlodipine + Diltiazem | 0.148 |
| Amlodipine | 0.228 | Amlodipine + Simvastatin | 0.148 |
| Diltiazem | 0.228 | Antidiabetics + Thyroxine | 0.148 |
| Methyldopa | 0.228 | Aspirin + Calcium | 0.148 |
| Atorvastatin | 0.482 | Aspirin + Diclofenac | 0.148 |
| Clopidogrel | 0.482 | Aspirin + Perindopril | 0.148 |
| Diclofenac | 0.482 | Aspirin + Prednisolone | 0.148 |
| Indapamide | 0.482 | Aspirin + Ramipril | 0.148 |
| Levothyroxine | 0.482 | Atorvastatin + Verapamil | 0.148 |
| Perindopril | 0.482 | Calcium acetate + Calcium aspartate | 0.148 |
| Prednisolone | 0.482 | Calcium + Indapamide | 0.148 |
| Ramipril | 0.482 | Calcium + Levothyroxine | 0.148 |
| Simvastatin | 0.482 | Calcium + NSAIDs | 0.148 |
| Thyroxine | 0.482 | Clopidogrel + NSAIDs | 0.148 |
| Pentoxifylline | 0.482 | Diltiazem + β-blockers | 0.148 |
| Pentoxifylline + NSAIDs | 0.148 |
| Diagnosis | Number of Patients | Avg. Number of Drug | Avg. Interaction Number |
|---|---|---|---|
| Hypertension | 77 (80.2%) | 6.4 | 1.6 |
| Diabetes mellitus | 24 (25.0%) | 7.0 | 2.6 |
| Hypertension + diabetes Mellitus | 17 (17.7%) | 7.1 | 2.7 |
| Other diseases | 12 (12.5%) | 5.3 | 0.8 |
| pDDIs | Number | Percent (%) |
|---|---|---|
| Decreased drug dffects | 38 | 25.45 |
| Hypertension | 35 | 23.45 |
| Hypotension | 27 | 18.05 |
| Arrhythmia | 15 | 10.05 |
| Bleeding risk | 11 | 7.50 |
| Hypoglycemia | 8 | 5.50 |
| Others | 15 | 10.00 |
| Total | 149 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahzadi, A.; Sonmez, I.; Kose, C.; Oktan, B.; Alagoz, S.; Sonmez, H.; Hussain, A.; Akkan, A.G. The Prevalence of Potential Drug-Drug Interactions in CKD-A Retrospective Observational Study of Cerrahpasa Nephrology Unit. Medicina 2022, 58, 183. https://doi.org/10.3390/medicina58020183
Shahzadi A, Sonmez I, Kose C, Oktan B, Alagoz S, Sonmez H, Hussain A, Akkan AG. The Prevalence of Potential Drug-Drug Interactions in CKD-A Retrospective Observational Study of Cerrahpasa Nephrology Unit. Medicina. 2022; 58(2):183. https://doi.org/10.3390/medicina58020183
Chicago/Turabian StyleShahzadi, Andleeb, Ikbal Sonmez, Cagla Kose, Burhaneddin Oktan, Selma Alagoz, Haktan Sonmez, Adil Hussain, and Ahmet Gokhan Akkan. 2022. "The Prevalence of Potential Drug-Drug Interactions in CKD-A Retrospective Observational Study of Cerrahpasa Nephrology Unit" Medicina 58, no. 2: 183. https://doi.org/10.3390/medicina58020183
APA StyleShahzadi, A., Sonmez, I., Kose, C., Oktan, B., Alagoz, S., Sonmez, H., Hussain, A., & Akkan, A. G. (2022). The Prevalence of Potential Drug-Drug Interactions in CKD-A Retrospective Observational Study of Cerrahpasa Nephrology Unit. Medicina, 58(2), 183. https://doi.org/10.3390/medicina58020183






