Evaluation of Dose-Dependent Obesity and Diabetes-Related Complications of Water Chestnut (Fruit of Trapa japonica) Extracts in Type II Obese Diabetic Mice Induced by 45% Kcal High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Material
2.2. HPLC Analyses
2.3. Experimental Animals
- Normal control mice (NFD-administered group);
- HFD control mice (HFD-administered group);
- HFD supply control group (metformin—250 mg/kg group);
- WC high dose (200 mg/kg), HFD-supplied experimental group;
- WC intermediate dose (100 mg/kg), HFD-supplied experimental group;
- WC low dose (50 mg/kg), HFD-supplied experimental group.
2.4. Selection of the Test Substance Dosage
2.5. Administration of the Test Substance
2.6. Changes in Body Weight
2.7. Measurement of Body Fat Density: Abdominal and Total Fat Mass (%)
2.8. Measurement of Blood Glucose Level
2.9. Serum Biochemical Analyses
2.10. Organ Weight Measurements
2.11. Lipid Composition Measurement in Feces
2.12. Measurement of Liver Lipid Peroxidation and Changes in Antioxidant Defense Systems
2.13. Hepatic Glucose-Regulating Enzyme Activities Measurement
2.14. Real-Time RT-PCR Analysis
2.15. Histopathology
2.16. Immunohistochemistry
2.17. Statistical Analyses
3. Results
3.1. Content of Ellagic Acid in WC Extract
3.2. Effects on Obesity
3.2.1. Change in Weight
3.2.2. Change in Amount of Average Feed Intake
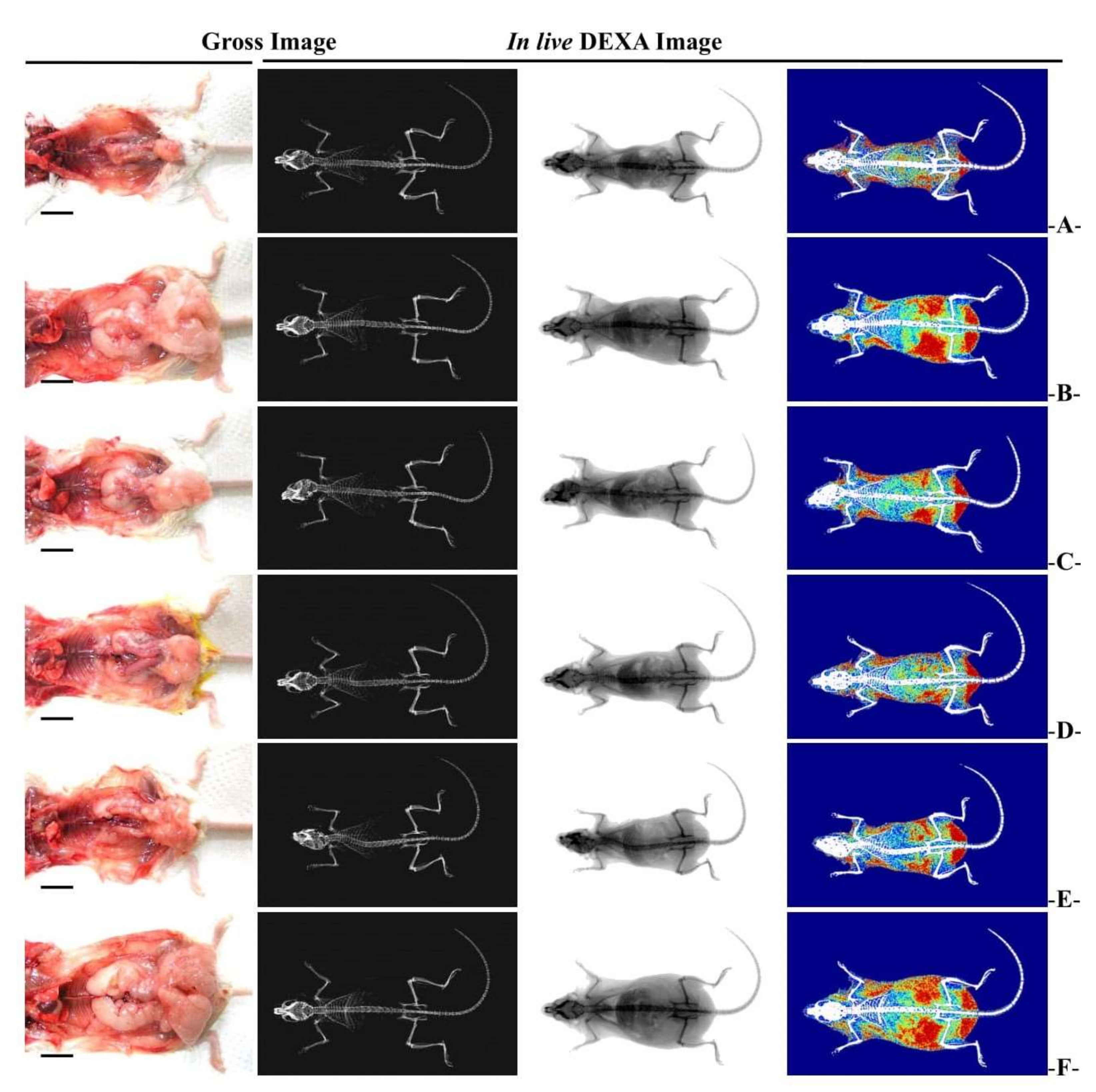
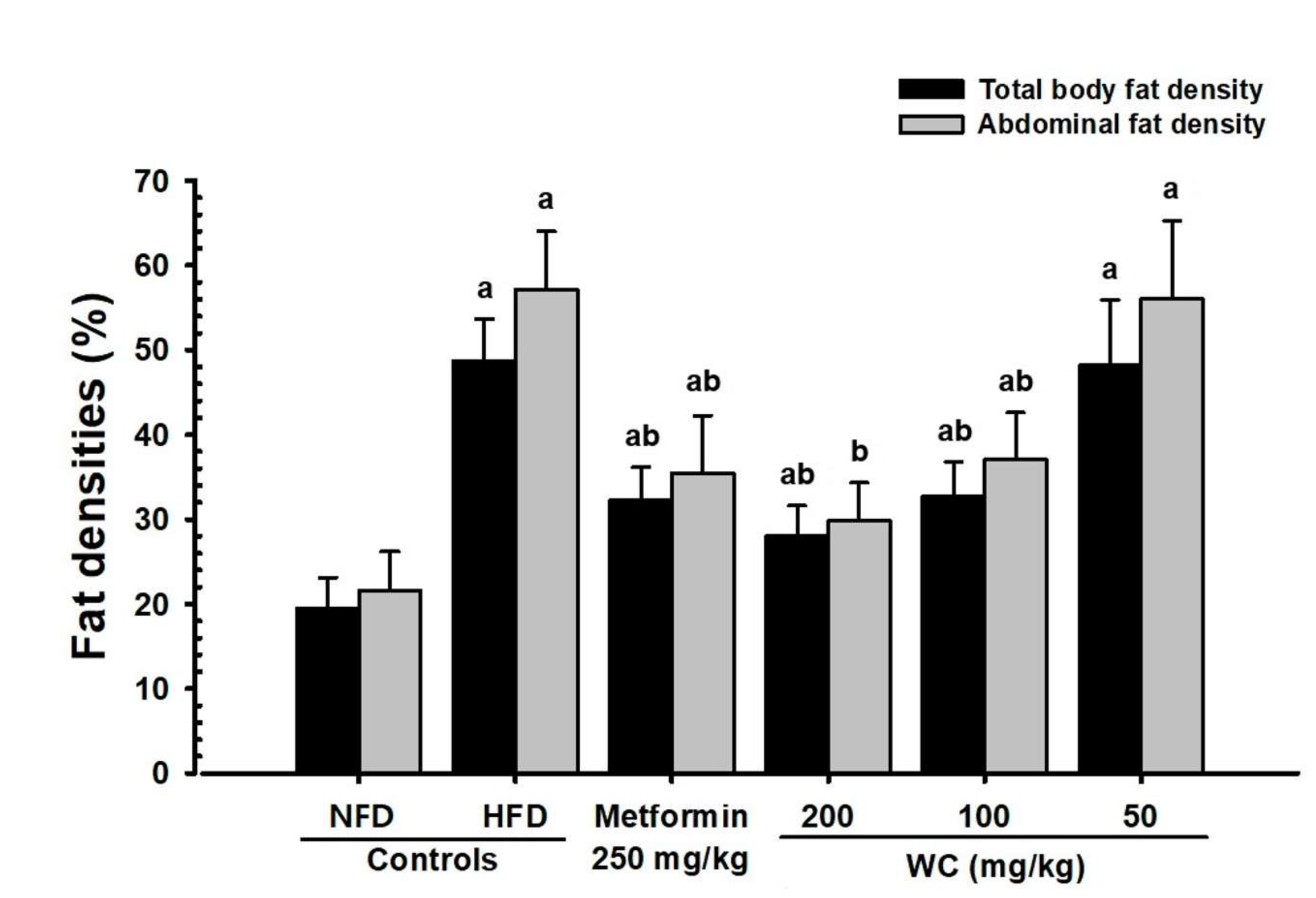
3.2.3. Changes in Abdominal Fat and Body Fat Mass
3.2.4. Change in Fat Weight
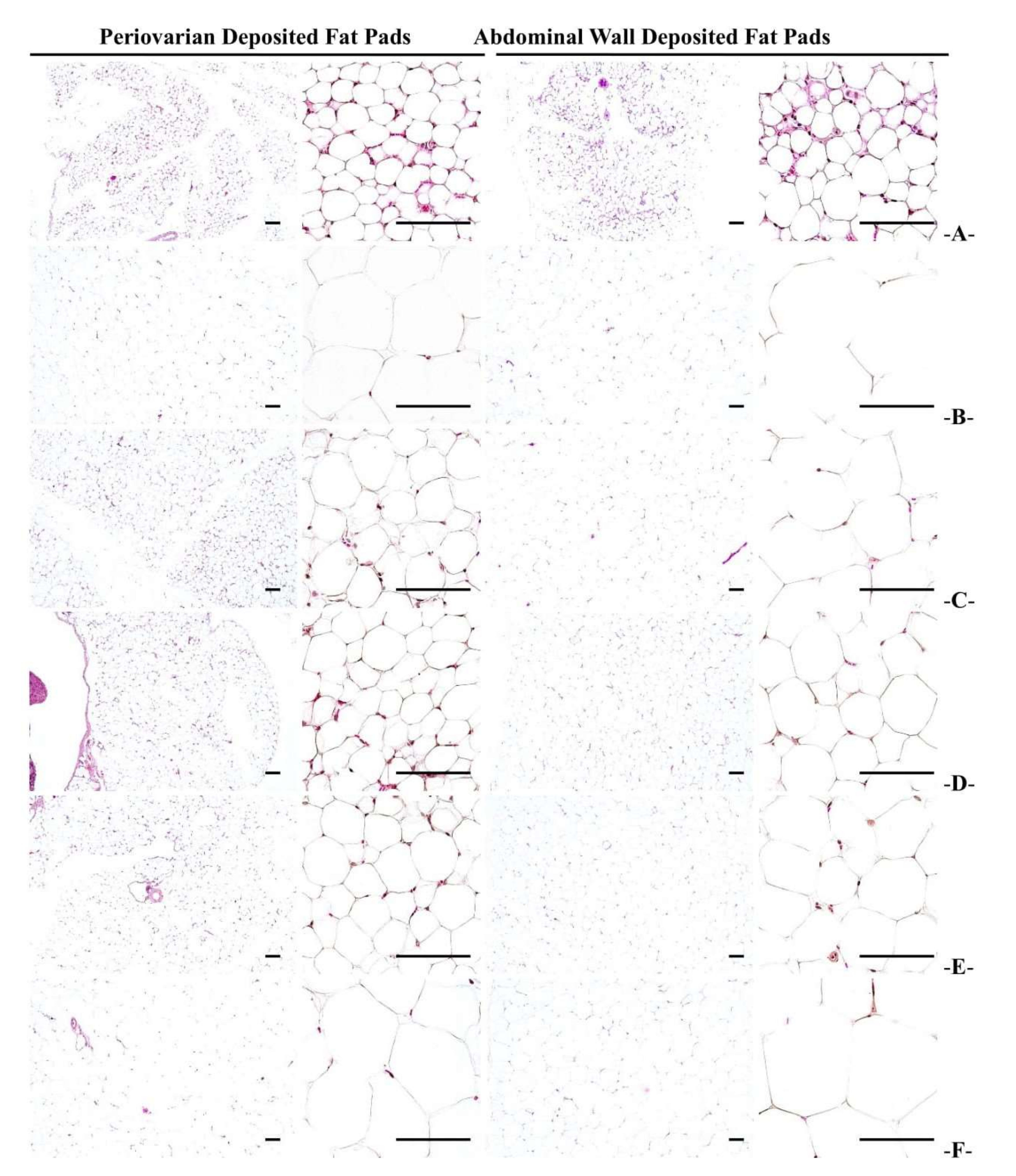
3.2.5. Histopathological Changes in Fat Accumulation around the Ovary and in the Abdominal Wall
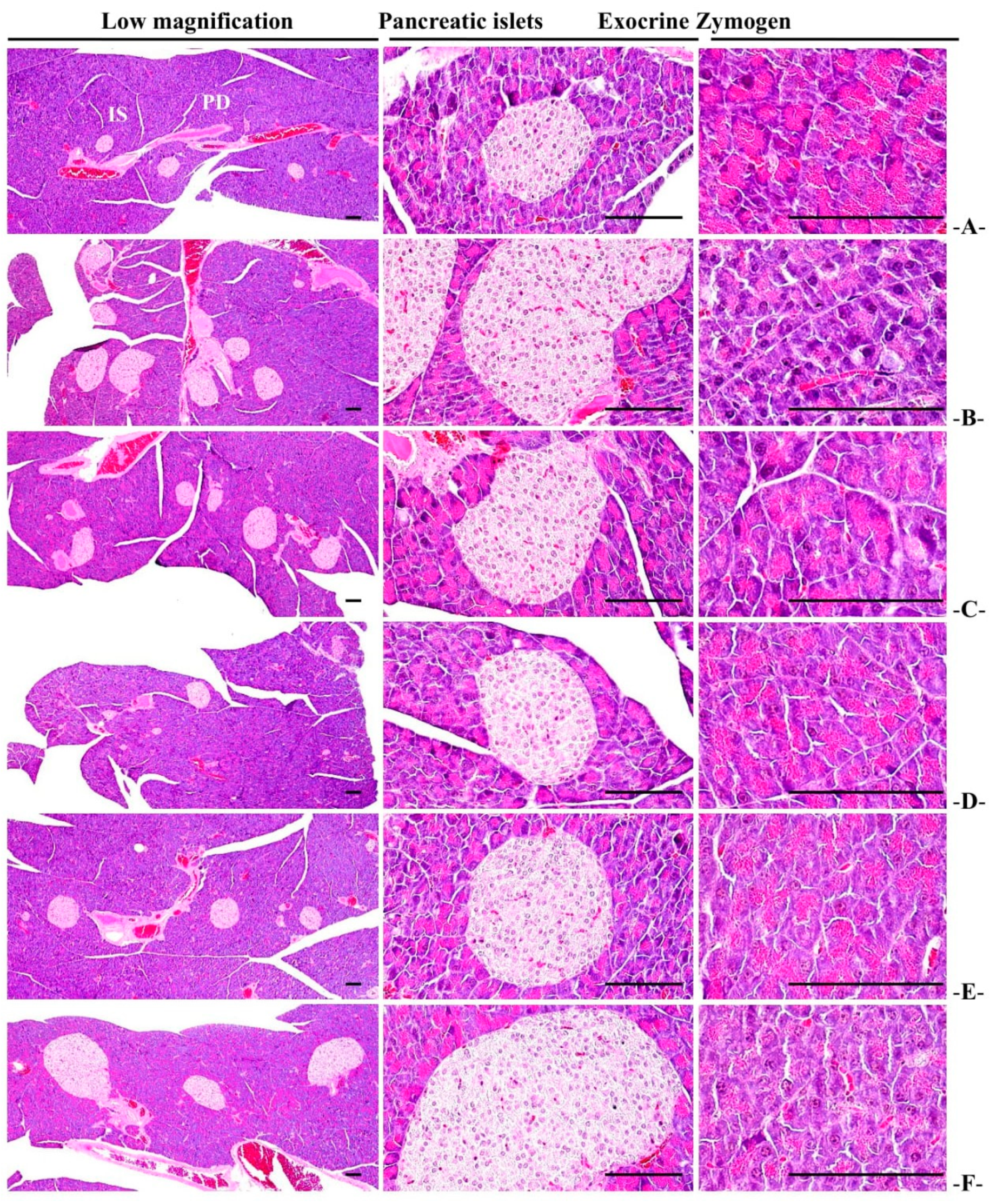
3.2.6. Histopathological Changes of Zymogen Granules in the Exocrine Pancreas
3.3. Antidiabetic Effect
3.3.1. Changes in Blood Sugar
3.3.2. Changes in Insulin Level in Blood
3.3.3. Changes in the Hba1c Ratio in Blood
3.3.4. Change in Pancreatic Weight
3.3.5. General Histopathological Changes in Pancreatic Islets
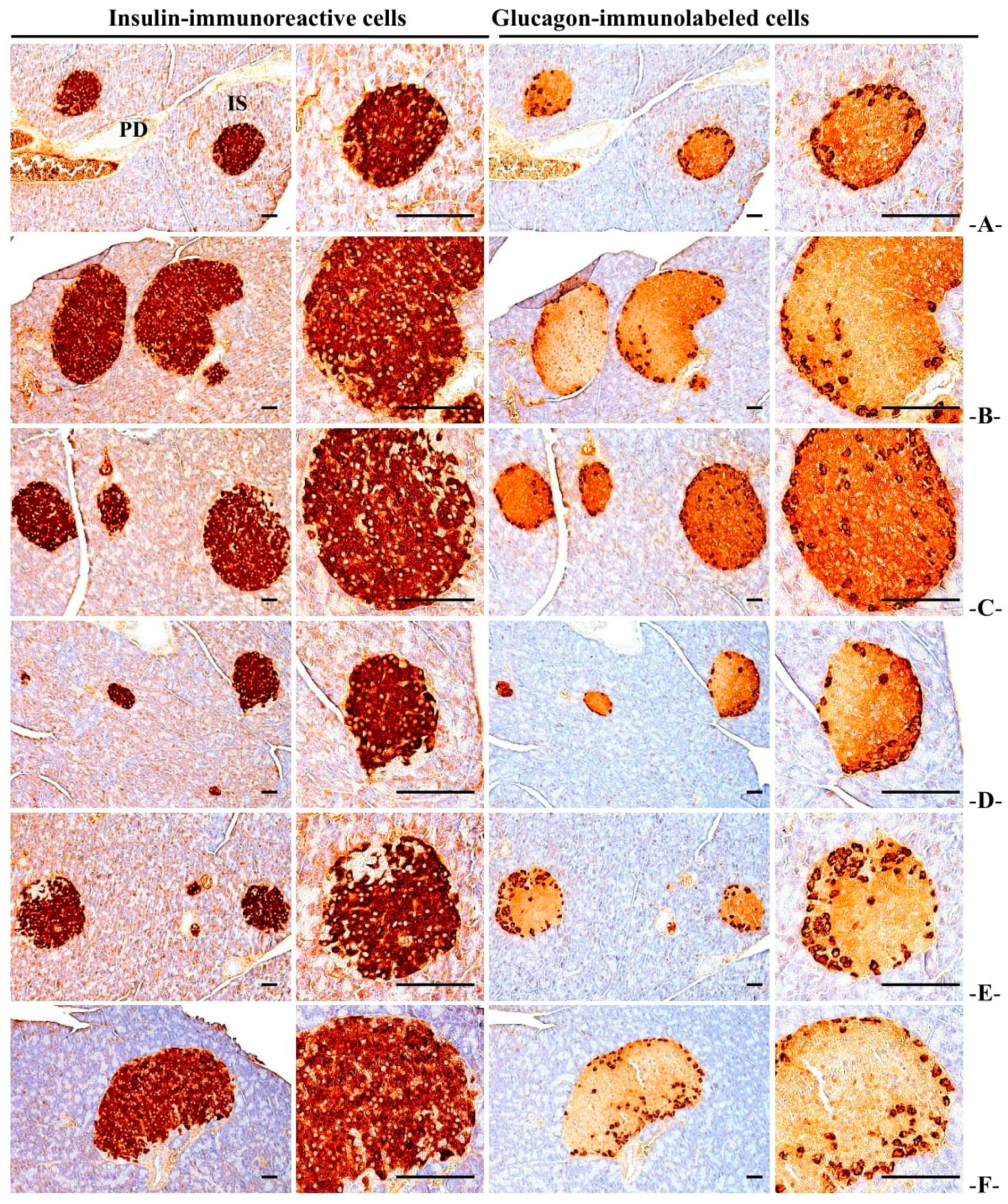
3.3.6. Immuno-Histochemical Changes in Pancreatic Islets
3.4. Effect on Hyperlipidemia
3.4.1. Changes in TG and Total Cholesterol Content in Blood
3.4.2. Changes in Blood HDL and LDL Content
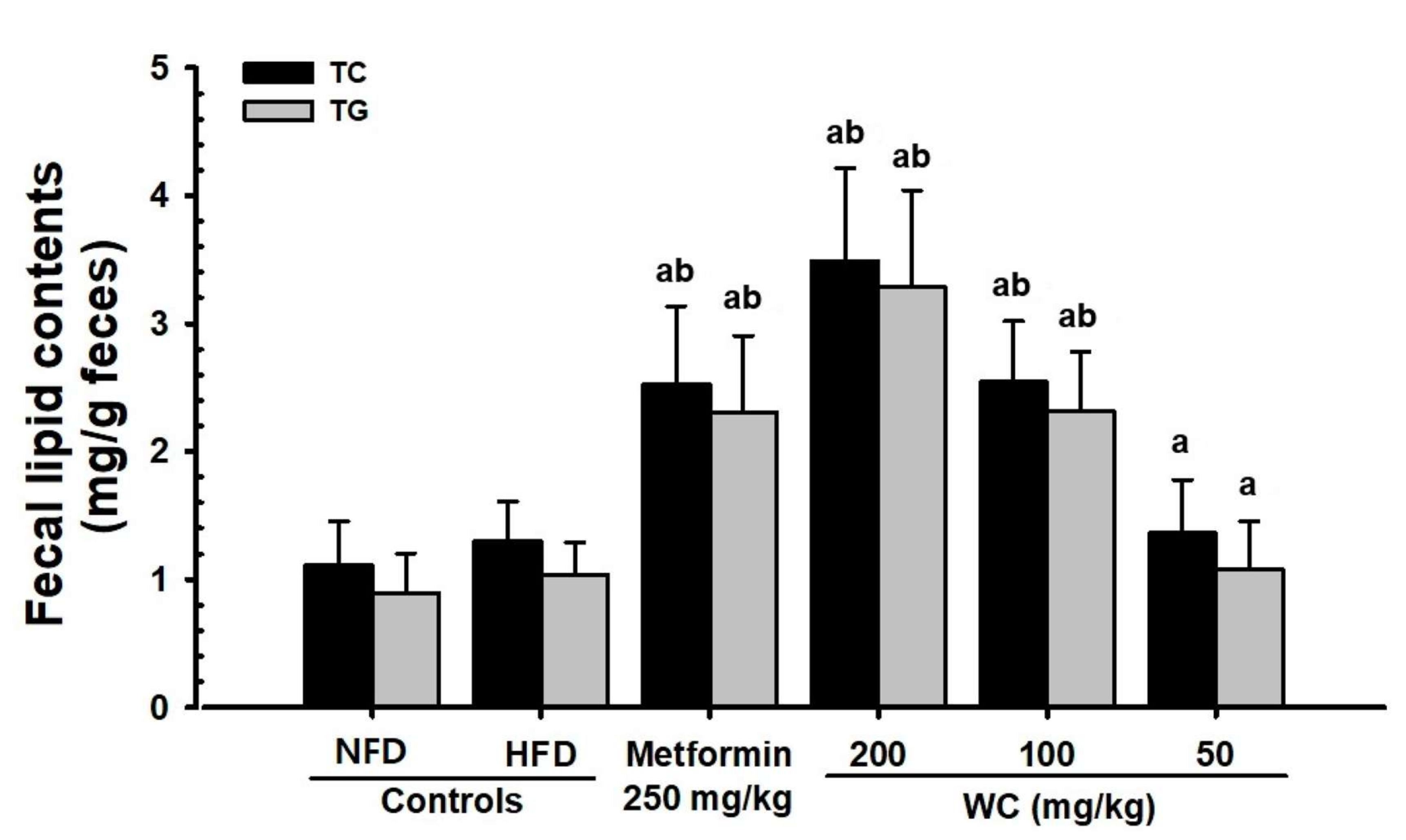
3.4.3. Changes in Lipid Content in Feces
3.5. Effects on Liver Damage
3.5.1. Change in Liver Weight
3.5.2. Changes in ALT, AST, GGT, LDH, and ALP Content in Blood
3.5.3. Histopathological Changes in Liver Fat Change Rate
3.5.4. Histopathological Changes in Liver Cell Diameter
3.6. Effects on Kidney Damage
3.6.1. Change in Height
3.6.2. Changes in Blood Creatinine and BUN Content
3.6.3. Renal Histopathological Changes
3.7. Effect on the Liver Antioxidant Defense System
3.7.1. Changes in Lipid Peroxidation
3.7.2. Changes in GSH Content in Liver Tissue
3.7.3. Changes in CAT and SOD Activity in Liver Tissue
3.8. Changes in Liver Enzyme Activity Related to Sugar Metabolism
3.8.1. Changes in GK Activity in Liver Tissue
3.8.2. Changes in G6Pase Activity in Liver Tissue
3.8.3. Changes in PEPCK Activity in Liver Tissue
3.9. Changes in Gene Expression Related to Lipid Metabolism
Changes in mRNA Expression in Liver Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsiki, N.; Athyros, V.G.; Karagiannis, A.; Mikhailidis, D.P. Characteristics other than the diagnostic criteria associated with metabolic syndrome: An overview. Curr. Vasc. Pharmacol. 2014, 12, 627–641. [Google Scholar] [CrossRef]
- Wendel, A.A.; Purushotham, A.; Liu, L.F.; Belury, M.A. Conjugated linoleic acid fails to worsen insulin resistance but induces hepatic steatosis in the presence of leptin in ob/ob mice. J. Lipid Res. 2008, 49, 98–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- James, P.T.; Leach, R.; Kalamara, E.; Shayeghi, M. The worldwide obesity epidemic. Obes. Res. 2001, 9, 228S–233S. [Google Scholar]
- World Health Organization. Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 5 December 2021).
- Lebovitz, H.E. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109, S135–S148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstein, B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. 2002, 90, 3G–10G. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.N.; Moon, S.J.; Ko, S.K.; Im, B.O.; Chung, S.H. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Arch. Pharm. Res. 2004, 27, 790–796. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.S.; Seol, D.J.; Cho, I.J.; Ku, S.K.; Choi, J.S.; Lee, H.J. Anti-obesity and fatty liver-preventing activities of Lonicera caerulea in high-fat diet-fed mice. Int. J. Mol. Med. 2018, 42, 3047–3064. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.R.; Kim, H.J.; Lee, Y.J.; Ku, S.K. Anti-diabetic obesity effects of Wasabia japonica Matsum leaf extract on 45% Kcal high-fat diet-fed mice. Nutrients 2020, 12, E2837. [Google Scholar] [CrossRef]
- Choi, E.H.; Chun, Y.S.; Kim, J.K.; Ku, S.K.; Jeon, S.W.; Park, T.S.; Shim, S.M. Modulating lipid and glucose metabolism by glycosylated kaempferol rich roasted leaves of Lycium chinense via upregulating adiponectin and AMPK activation in obese mice-induced type 2 diabetes. J. Funct. Foods 2020, 72, 104072. [Google Scholar] [CrossRef]
- Sone, H.; Suzuki, H.; Takahashi, A.; Yamada, N. Disease model: Hyperinsulinemia and insulin resistance. Part A-targeted disruption of insulin signaling or glucose transport. Trends Mol. Med. 2001, 7, 320–322. [Google Scholar] [CrossRef]
- Ku, S.K.; Sung, S.H.; Choung, J.J.; Choi, J.-S.; Shin, Y.K.; Kim, J.W. Anti-obesity and anti-diabetic effects of a standardized potato extract in ob/ob mice. Exp. Ther. Med. 2016, 12, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Inzucchi, S.E. Oral antihyperglycemic therapy for type 2 diabetes: Scientific review. JAMA 2002, 287, 360–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Quz, Z.; Fu, L.; Dong, P.; Zhang, X. Physicochemical properties and antioxidant capacity of 3 polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2009, 74, C469–C474. [Google Scholar] [CrossRef] [PubMed]
- Hays, N.P.; Galassetti, P.R.; Coker, R.H. Prevention and treatment of type 2 diabetes: Current role of lifestyle, natural product, and pharmacological interventions. Pharmacol. Ther. 2008, 118, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.I.; Apostolidis, E.; Shetty, K. In vitro studies of eggplant (Solanum melongena) phenolics as inhibitors of key enzymes relevant for type 2 diabetes and hypertension. Bioresour. Technol. 2008, 99, 2981–2988. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Wang, J.; Yang, L.; An, Y.; Zhu, H. AMPK activation enhances the anti-atherogenic effects of high density lipoproteins in apoE(−/−) mice. J. Lipid Res. 2017, 58, 1536–1547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottillo, E.P.; Desjardins, E.M.; Fritzen, A.M.; Zou, V.Z.; Crane, J.D.; Yabut, J.M.; Kiens, B.; Erion, D.M.; Lanba, A.; Granneman, J.G.; et al. FGF21 does not require adipocyte AMP-activated protein kinase (AMPK) or the phosphorylation of acetyl-CoA carboxylase (ACC) to mediate improvements in whole-body glucose homeostasis. Mol. Metab. 2017, 6, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.B.; Peters, A.L. An overview of metformin in the treatment of type 2 diabetes mellitus. Am. J. Med. 1997, 102, 99–110. [Google Scholar] [CrossRef]
- Jang, J.D.; Kim, M.; Nam, G.H.; Kim, Y.M.; Kang, S.M.; Lee, K.Y.; Park, Y.J. Antiaging activity of peptide identified from fermented Trapa Japonica fruit extract in human dermal fibroblasts. Evid. Based Complement. Altern. Med. 2020, 2020, 5895029. [Google Scholar]
- Yasuda, M.; Yasutake, K.; Hino, M.; Ohwatari, H.; Ohmagari, N.; Takedomi, K.; Tanaka, T.; Nonaka, G. Inhibitory effects of polyphenols from water chestnut (Trapa japonica) husk on glycolytic enzymes and postprandial blood glucose elevation in mice. Food Chem. 2014, 165, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Kim, J.E.; Choi, B.K.; Kim, H.S. Anti-Inflammatory Effects of water chestnut extract on cytokine responses via nuclear factor-κB-signaling pathway. Biomol. Ther. 2015, 23, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Hwang, J.W.; Jang, J.H.; Son, S.; Seo, I.B.; Jeong, J.H.; Kim, E.H.; Moon, S.H.; Jeon, B.T.; Park, P.J. Trapa japonica pericarp extract reduces LPS-induced inflammation in macrophages and acute lung injury in mice. Molecules 2016, 21, 392. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.R.; Ciou, J.Y.; Chiang, P.Y. Effect of micronization on functional properties of the water caltrop (Trapa taiwanensis Nakai) pericarp. Food Chem. 2009, 113, 970–974. [Google Scholar] [CrossRef]
- Shindo, K.; Kuroki, E.; Toyoda, M. Antioxidative compounds contained in the seed with hard shell of Trapa japonica Flerov. and its herbal tea. J. Home Econ. Jpn. 2013, 64, 353–359. [Google Scholar]
- Lee, D.; Lee, O.H.; Choi, G.; Kim, J.D. Antioxidant and anti-adipogenic activities of Trapa japonica shell extract cultivated in Korea. Prev. Nutr. Food Sci. 2017, 22, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Hwang, Y.; Hwang, B.S.; Shin, S.Y.; Cho, P.Y.; Lee, S.Y.; Choi, K.M.; Lee, K.W.; Kim, G.Y.; Cho, Y.H.; et al. Trapa japonica inhibits adipocyte differentiation and adipogenesis through AMPK signaling pathway in 3T3-L1 pre-adipocytes. Int. J. Food Sci. Nutr. 2019, 1, 1008. [Google Scholar] [CrossRef]
- Kang, M.J.; Lee, S.K.; Song, J.H.; Kim, M.E.; Kim, M.J.; Jang, J.S.; Lee, J.H.; Kim, J.I. Water chestnut (Trapa japonica Flerov.) exerts inhibitory effect on postprandial glycemic response in rats and free radical scavenging activity in vitro. Food Sci. Biotechnol. 2009, 18, 808–812. [Google Scholar]
- Seufert, J.; Lubben, G.; Dietrich, K.; Bates, P.C. A comparison of the effects of thiazolidinediones and metformin on metabolic control in patients with type 2 diabetes mellitus. Clin. Ther. 2004, 26, 805–818. [Google Scholar]
- Park, S.H.; Ko, S.K.; Chung, S.H. Euonymus alatus prevents the hyperglycemia and hyperlipidemia induced by high-fat diet in ICR mice. J. Ethnopharmacol. 2005, 102, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Song, C.-H.; Park, S.-J.; Park, D.-C.; Cho, H.-R.; Jung, G.-W.; Bashir, K.M.I.; Ku, S.K.; Choi, J.-S. Protective effects of triple fermented barley extracts (FBe) on HCl/EtOH-induced gastric mucosa damages in mice. Food Sci. Nutr. 2018, 6, 2036–2046. [Google Scholar] [CrossRef]
- Morton, D.B.; Griffiths, P.H. Guidelines on the recognition of pain, distress and discomfort in experimental animals and an hypothesis for assessment. Vet. Rec. 1985, 116, 431–436. [Google Scholar] [CrossRef]
- Flecknell, P. Laboratory Animal Anesthesia, 2nd ed.; Harcourt Brace & Company: San Diego, CA, USA, 1996; pp. 1–269. [Google Scholar]
- Korea Food and Drug Administration. Testing Guidelines for Safety Evaluation of Drugs; Notification No. 2017-071; Korea Food and Drug Administration: Seoul, Korea, 2017. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Kavutcu, M.; Canbolat, O.; Oztürk, S.; Olcay, E.; Ulutepe, S.; Ekinci, C.; Gökhun, I.H.; Durak, I. Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin-treated guinea pigs: Effects of vitamins E and C. Nephron 1996, 72, 269–274. [Google Scholar] [CrossRef]
- Jamall, I.S.; Smith, J.C. Effects of cadmium on glutathione peroxidase, superoxidase dismutase and lipid peroxidation in the rat heart: A possible mechanism of cadmium cardiotoxicity. Toxicol. Appl. Pharmacol. 1985, 80, 33–42. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. In Methods in Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–686. [Google Scholar]
- Sun, Y.; Larry, W.O.; Ying, L.A. Simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988, 34, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Hulcher, F.H.; Oleson, W.H. Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J. Lipid Res. 1973, 14, 625–631. [Google Scholar] [CrossRef]
- Davidson, A.L.; Arion, W.J. Factors underlying significant underestimations of glucokinase activity in crude liver extracts: Physiological implications of higher cellular activity. Arch. Biochem. Biophys. 1987, 253, 156–167. [Google Scholar] [CrossRef]
- Alegre, M.; Ciudad, C.J.; Fillat, C.; Guinovart, J.J. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal. Biochem. 1988, 173, 185–189. [Google Scholar] [CrossRef]
- Bentle, L.A.; Lardy, H.A. Interaction of anions and divalent metal ions with phosphoenolpyruvate carboxykinase. J. Biol. Chem. 1976, 251, 2916–2921. [Google Scholar] [CrossRef]
- Veiga, F.M.S.; Graus-Nunes, F.; Rachid, T.L.; Barreto, A.B.; Mandarim-de-Lacerda, C.A.; Souza-Mello, V. Anti-obesogenic effects of WY14643 (PPAR-alpha agonist): Hepatic mitochondrial enhancement and suppressed lipogenic pathway in diet-induced obese mice. Biochimie 2017, 140, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Kim, D.S.; Kim, S.H.; Kim, H.K. Anti-obesity activity, acute toxicity, and chemical constituents of aqueous and ethanol Viola mandshurica extracts. BMC Complement. Altern. Med. 2017, 17, 297. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Levene, A. Pathological factors influencing excision of tumours in the head and neck. Part I. Clin. Otolaryngol. Allied Sci. 1981, 6, 145–151. [Google Scholar] [CrossRef]
- Williams, C.D.; Stengel, J.; Asike, M.I.; Torres, D.M.; Shaw, J.; Contreras, M.; Landt, C.L.; Harrison, S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middleaged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology 2011, 140, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Kim, J.; Cheng, J.; Ong, M.; Lao, W.G.; Jin, X.L.; Lin, Y.G.; Xiao, L.; Zhu, X.Q.; Qu, X.Q. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J. Gastroenterol. 2017, 23, 3805–3814. [Google Scholar] [CrossRef]
- Pais, R.; Charlotte, F.; Fedchuk, L.; Bedossa, P.; Lebray, P.; Poynard, T.; Ratziu, V. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013, 59, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Rotman, Y.; Sanyal, A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 2017, 66, 180–190. [Google Scholar] [CrossRef] [Green Version]
- Gartner, L.P.; Hiatt, J.L. Color Textbook of Histology, 3rd ed.; Saunders: Philadelphia, PA, USA, 2007; pp. 417–422. [Google Scholar]
- Wilson, J.S.; Korsten, M.A.; Leo, M.A.; Lieber, C.S. Combined effects of protein deficiency and chronic ethanol consumption on rat pancreas. Dig. Dis. Sci. 1988, 33, 1250–1259. [Google Scholar] [CrossRef]
- Bookchin, R.M.; Gallop, P.M. Structure of hemoglobin AIc; nature of the N-terminal beta chain blocking group. Biochem. Biophys. Res. Commun. 1968, 32, 86–93. [Google Scholar] [CrossRef]
- Larsen, M.L.; Hørder, M.; Mogensen, E.F. Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1990, 323, 1021–1025. [Google Scholar] [CrossRef]
- Sodikoff, C.H. Laboratory Profiles of Small Animal Diseases: A Guide to Laboratory Diagnosis, 2nd ed.; Mosby: St. Louise, MO, USA, 1995; pp. 1–36. [Google Scholar]
- Garg, M.C.; Singh, K.P.; Bansal, D.D. Effect of vitamin C supplementation on oxidative stress in experimental diabetes. Indian J. Exp. Biol. 1997, 35, 264–266. [Google Scholar]
- Giugliano, D.; Ceriello, A.; Paolisso, G. Oxidative stress and diabetic vascular complications. Diabetes Care 1996, 19, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Comporti, M. Lipid peroxidation and cellular damage in toxic liver injury. Lab. Investig. 1985, 53, 599–623. [Google Scholar]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.; Salam, S.K.; Salleh, M.S.; Gurtu, S. Comparison of antioxidant effects of honey, glibenclamide, metformin, and their combinations in the kidneys of streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2011, 12, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.I.; Rico, C.W.; Kang, M.Y. Comparative study on the hypoglycemic and antioxidative effects of fermented paste (doenjang) prepared from soybean and brown rice mixed with rice bran or red ginseng marc in mice fed with high fat diet. Nutrients 2014, 6, 4610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Zheng, N.; Qi, K.; Cheng, H.; Sun, Z.; Gao, B.; Zhang, Y.; Pang, W.; Huangfu, C.; Ji, S.; et al. Exogenous hydrogen sulfide mitigates the fatty liver in obese mice through improving lipid metabolism and antioxidant potential. Med. Gas Res. 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferre, T.; Riu, E.; Bosch, F.; Valera, A. Evidence from transgenic mice that glucokinase is rate limiting for glucose utilization in the liver. FASEB J. 1996, 10, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Coope, G.J.; Atkinson, A.M.; Allott, C.; McKerrecher, D.; Johnstone, C.; Pike, K.G.; Holme, P.C.; Vertigan, H.; Gill, D.; Coghlan, M.P.; et al. Predictive blood glucose lowering efficacy by Glucokinase activators in high fat fed female Zucker rats. Br. J. Pharmacol. 2006, 149, 328–335. [Google Scholar] [CrossRef] [Green Version]
- She, P.; Shiota, M.; Shelton, K.D.; Chalkley, R.; Postic, C.; Magnuson, M.A. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol. Cell. Biol. 2000, 20, 6508–6517. [Google Scholar] [CrossRef]
- Van Schaftingen, E.; Gerin, I. The glucose-6-phosphatase system. Biochem. J. 2002, 362, 513–532. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Effects of Bofu-Tsusho-San on diabetes and hyperlipidemia associated with AMP-activated protein kinase and glucose transporter 4 in high-fat-fed mice. Int. J. Mol. Sci. 2014, 15, 22. [Google Scholar] [CrossRef] [Green Version]
- Malini, P.; Kanchana, G.; Rajadurai, M.U.R.U. Antidiabetic efficacy of ellagic acid in streptozotocin-induced diabetes mellitus in albino wistar rats. Asian J. Pharm. Clin. Res. 2011, 4, 124–128. [Google Scholar]
- Ahad, A.; Ganai, A.A.; Mujeeb, M.; Siddiqui, W.A. Ellagic acid, an NF-κB inhibitor, ameliorates renal function in experimental diabetic nephropathy. Chem.-Biol. Interact. 2014, 219, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Farbood, Y.; Rashno, M.; Ghaderi, S.; Khoshnam, S.E.; Sarkaki, A.; Rashidi, K.; Rashno, M.; Badavi, M. Ellagic acid protects against diabetes-associated behavioral deficits in rats: Possible involved mechanisms. Life Sci. 2019, 225, 8–19. [Google Scholar] [CrossRef] [PubMed]






| Compositions | High-Fat Diets | Normal Pellet Diets |
|---|---|---|
| Ingredient (g/kg) | ||
| Sucrose | 172.8 | 500 |
| Casein | 200 | 200 |
| Lard | 177.5 | 0 |
| Corn starch | 72.8 | 150 |
| Cellulose | 50 | 50 |
| Mineral mixture | 35 | 35 |
| Soybean Oil | 25 | 50 |
| Vitamin mixture | 10 | 10 |
| Choline bitartrate | 2 | 2 |
| L-Cystein | 3 | 3 |
| Fat (% kcal) | 45 | 16 |
| Carbohydrate (% kcal) | 35 | 64 |
| Protein (% kcal) | 20 | 20 |
| Energy (kcal/g) | 4.73 | 4.00 |
| Target | 5′–3′ | Sequence | GenBank Accession Number |
|---|---|---|---|
| PPARα | Forward Reverse | ATGCCAGTACTGCCGTTTTC GGCCTTGACCTTGTTCATGT | NM_011144 |
| PPARγ | Forward Reverse | AGTGGAGACCGCCCAGG GCAGCAGGTTGTCTTGGATGT | NM_001127330 |
| Leptin | Sense Antisense | CCAAAACCCTCATCAAGACC GTCCAACTGTTGAAGAATGTCCC | NM_008493 |
| UCP2 | Sense Antisense | CCGCATTGGCCTCTACGACTCT CCCCGAAGGCAGAAGTGAAGTG | NM_011671 |
| Adiponectin | Sense Antisense | CCCAAGGGAACTTGTGCAGGTTGGATG GTTGGTATCATGGTAGAGAAGAAAGCC | NM_009605 |
| C/EBPα | Sense Antisense | TGGACAAGAACAGCAACGAGTAC CGGTCATTGTCACTGGTCAACT | NM_001287523 |
| C/EBPβ | Sense Antisense | AAGCTGAGCGACGAGTACAAGA GTCAGCTCCAGCACCTTGTG | NM_001287739 |
| SREBP1c | Sense Antisense | AGCCTGGCCATCTGTGAGAA CAGACTGGTACGGGCCACAA | XM_006532714 |
| FAS | Sense Antisense | GCTGCGGAAACTTCAGGAAAT AGAGACGTGTCACTCCTGGACTT | NM_007988 |
| ACC1 | Sense Antisense | GCCATTGGTATTGGGGCTTAC CCCGACCAAGGACTTTGTTG | NM_133360 |
| AMPKα1 | Sense Antisense | AAGCCGACCCAATGACATCA CTTCCTTCGTACACGCAAAT | XM_011245321 |
| AMPKα2 | Sense Antisense | GATGATGAGGTGGTGGA GCCGAGGACAAAGTGC | NM_178143 |
| GAPDH | Sense Antisense | CATCTTCCAGGAGCGAGACC TCCACCACCCTGTTGCTGTA | NM_008084 |
| Times Groups | Body Weights (g) at Days after Initial Test Substance Treatment | Body Weight Gains during | Mean Daily Food Consumption (g) | ||||
|---|---|---|---|---|---|---|---|
| 8 Days before (A) | 1 Day before (B) | 0 Day * (C) | 84 Days * (D) | Adapt Period (B−A) | Administration Period (D−C) | ||
| Controls | |||||||
| NFD | 29.41 ± 1.52 | 30.65 ± 1.84 | 26.72 ± 2.00 | 31.09 ± 1.73 | 1.24 ± 0.87 | 4.37 ± 1.23 | 4.94 ± 0.60 |
| HFD | 29.68 ± 0.89 | 33.98 ± 1.94 a | 30.81 ± 1.57 a | 44.02 ± 2.74 a | 4.30 ± 1.97 a | 13.21 ± 2.72 a | 4.31 ± 0.73 b |
| Reference | |||||||
| Metformin | 29.66 ± 1.49 | 33.93 ± 1.64 a | 30.79 ± 1.61 a | 36.68 ± 2.30 ac | 4.27 ± 0.91 a | 5.89 ± 2.05 c | 4.35 ± 0.64 b |
| Test substances—WC | |||||||
| 200 mg/kg | 29.02 ± 1.47 | 33.39 ± 1.38 a | 30.45 ± 1.26 a | 34.61 ± 1.85 bc | 4.37 ± 1.18 a | 4.16 ± 1.94 c | 4.31 ± 0.66 b |
| 100 mg/kg | 29.13 ± 1.59 | 33.45 ± 1.59 a | 30.26 ± 1.85 a | 36.03 ± 2.73 ac | 4.32 ± 1.16 a | 5.77 ± 2.09 c | 4.31 ± 0.72 b |
| 50 mg/kg | 29.23 ± 1.13 | 33.56 ± 1.41 a | 30.21 ± 1.79 a | 43.00 ± 2.41 a | 4.33 ± 0.62 a | 12.79 ± 2.69 a | 4.36 ± 0.64 b |
| Organs Groups | Absolute Organ Weights (g) | ||||
|---|---|---|---|---|---|
| Liver | Kidney | Pancreas | Periovarian Fat Pads | Abdominal Wall Fat Pads | |
| Controls | |||||
| NFD | 1.269 ± 0.064 | 0.195 ± 0.018 | 0.240 ± 0.045 | 0.072 ± 0.024 | 0.063 ± 0.030 |
| HFD | 1.887 ± 0.115 a | 0.289 ± 0.016 a | 0.232 ± 0.035 | 0.643 ± 0.116 c | 0.599 ± 0.080 a |
| Reference | |||||
| Metformin | 1.548 ± 0.103 ab | 0.239 ± 0.017 ab | 0.253 ± 0.033 | 0.302 ± 0.074 cd | 0.291 ± 0.062 ab |
| Test materials—WC | |||||
| 200 mg/kg | 1.393 ± 0.152 b | 0.213 ± 0.016 b | 0.258 ± 0.042 | 0.219 ± 0.116 cd | 0.203 ± 0.080 ab |
| 100 mg/kg | 1.548 ± 0.115 ab | 0.234 ± 0.018 ab | 0.250 ± 0.041 | 0.301 ± 0.053 cd | 0.293 ± 0.051 ab |
| 50 mg/kg | 1.840 ± 0.139 a | 0.280 ± 0.023 ab | 0.245 ± 0.036 | 0.624 ± 0.119 c | 0.564 ± 0.099 a |
| Organs Groups | Relative Organ Weights (% of Body Weights) | ||||
| Liver | Kidney | Pancreas | Periovarian Fat Pads | Abdominal wall Fat Pads | |
| Controls | |||||
| NFD | 4.091 ± 0.299 | 0.628 ± 0.047 | 0.770 ± 0.129 | 0.229 ± 0.069 | 0.200 ± 0.092 |
| HFD | 4.294 ± 0.281 | 0.657 ± 0.034 | 0.526 ± 0.074 a | 1.466 ± 0.285 c | 1.362 ± 0.176 a |
| Reference | |||||
| Metformin | 4.230 ± 0.323 | 0.654 ± 0.063 | 0.690 ± 0.074 b | 0.826 ± 0.208 cd | 0.796 ± 0.167 ab |
| Test materials–WC | |||||
| 200 mg/kg | 4.034 ± 0.482 | 0.618 ± 0.063 | 0.747 ± 0.117 b | 0.624 ± 0.302 cd | 0.581 ± 0.203 ab |
| 100 mg/kg | 4.318 ± 0.462 | 0.653 ± 0.067 | 0.695 ± 0.112 b | 0.837 ± 0.143 cd | 0.817 ± 0.149 ab |
| 50 mg/kg | 4.294 ± 0.441 | 0.653 ± 0.067 | 0.571 ± 0.090 a | 1.451 ± 0.274 c | 1.307 ± 0.187 a |
| Items Groups | Periovarian Fat Pads | Abdominal Wall Fat Pads | ||
|---|---|---|---|---|
| Thickness (mm) | Adipocyte Diameters (μm) | Thickness (mm) | Adipocyte Diameters (μm) | |
| Controls | ||||
| NFD | 1.43 ± 0.55 | 31.91 ± 6.49 | 1.17 ± 0.62 | 37.19 ± 6.42 |
| HFD | 5.81 ± 0.91 a | 99.26 ± 14.38 a | 6.77 ± 1.18 a | 131.31 ± 21.03 d |
| Reference | ||||
| Metformin | 3.34 ± 0.67 ac | 55.82 ± 12.68 ac | 3.81 ± 0.67 ac | 75.34 ± 11.65 de |
| Test materials–WC | ||||
| 200 mg/kg | 2.65 ± 0.42 bc | 42.65 ± 10.41 c | 2.80 ± 0.67 ac | 59.45 ± 13.80 de |
| 100 mg/kg | 3.35 ± 0.71 ac | 55.65 ± 11.59 ac | 3.83 ± 0.78 ac | 75.98 ± 14.69 de |
| 50 mg/kg | 5.65 ± 1.46 a | 96.80 ± 20.55 a | 6.62 ± 1.51 a | 127.61 ± 22.50 d |
| Items Groups | Zymogen Granules (%/mm2 of Exocrine) | Mean Islet Numbers (Numbers/10 mm2) | Mean Islet Diamete (μm/Islet) | Insulin-IR Cells (cells/mm2) (A) | Glucagon-IR Cells (cells/mm2) (B) | Insulin/Glucagon Ratio (A/B) |
|---|---|---|---|---|---|---|
| Controls | ||||||
| NFD | 57.80 ± 11.34 | 5.00 ± 1.49 | 111.04 ± 14.01 | 230.00 ± 50.24 | 71.80 ± 15.54 | 3.20 ± 0.16 |
| HFD | 11.46 ± 3.34 c | 20.00 ± 1.89 c | 228.10 ± 30.15 c | 2801.60 ± 270.08 c | 439.40 ± 59.40 a | 6.43 ± 0.62 c |
| Reference | ||||||
| Metformin | 27.37 ± 4.88 cd | 11.80 ± 2.90 cd | 168.55 ± 15.66 cd | 1207.50 ± 261.04 cd | 288.10 ± 64.21 ab | 4.20 ± 0.12 cd |
| Test materials—WC | ||||||
| 200 mg/kg | 38.76 ± 7.97 cd | 8.10 ± 1.52 cd | 148.70 ± 14.65 cd | 737.70 ± 157.23 cd | 204.90 ± 47.80 ab | 3.61 ± 0.10 cd |
| 100 mg/kg | 27.13 ± 6.38 cd | 11.90 ± 2.88 cd | 168.42 ± 12.47 cd | 1243.40 ± 252.14 cd | 289.40 ± 55.85 ab | 4.29 ± 0.13 cd |
| 50 mg/kg | 11.75 ± 4.91 c | 19.20 ± 3.39 c | 222.91 ± 37.97 c | 2728.10 ± 532.60 c | 429.10 ± 85.38 a | 6.36 ± 0.19 c |
| Items Groups | Glucose (mg/dL) | Total Cholesterol (mg/dL) | Triglyceride (mg/dL) | Low-Density Lipoprotein (mg/dL) | High-Density Lipoprotein (mg/dL) |
|---|---|---|---|---|---|
| Controls | |||||
| NFD | 90.90 ± 11.82 | 89.30 ± 12.01 | 74.50 ± 10.46 | 17.40 ± 3.50 | 94.80 ± 17.84 |
| HFD | 258.70 ± 57.74 d | 278.40 ± 31.21 d | 252.50 ± 53.96 a | 87.10 ± 16.82 a | 23.30 ± 6.04 a |
| Reference | |||||
| Metformin | 141.70 ± 15.41 de | 162.40 ± 24.97 de | 145.90 ± 17.63 ac | 50.30 ± 14.01 ac | 49.50 ± 10.37 ac |
| Test materials—WC | |||||
| 200 mg/kg | 111.30 ± 17.54 de | 123.30 ± 17.96 de | 112.80 ± 30.68 c | 38.80 ± 14.16 bc | 59.40 ± 17.72 ac |
| 100 mg/kg | 140.40 ± 22.02 de | 161.30 ± 24.45 de | 145.70 ± 26.38 ac | 49.90 ± 11.26 ac | 49.70 ± 13.22 ac |
| 50 mg/kg | 252.70 ± 65.34 d | 268.40 ± 46.15 d | 247.40 ± 59.53 a | 83.10 ± 22.99 a | 24.10 ± 11.62 a |
| Items Groups | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | LDH (×10 IU/L) | GGT (IU/L) | BUN (mg/dL) | Creatinine (mg/dL) |
|---|---|---|---|---|---|---|---|
| Controls | |||||||
| NFD | 82.20 ± 14.51 | 45.60 ± 14.51 | 73.00 ± 15.04 | 51.62 ± 14.19 | 4.30 ± 2.21 | 31.80 ± 11.68 | 0.60 ± 0.16 |
| HFD | 219.40 ± 40.68 d | 155.90 ± 14.98 a | 214.60 ± 46.61 d | 404.42 ± 107.31 d | 16.40 ± 2.84 a | 111.00 ± 18.15 d | 2.14 ± 0.40 a |
| Reference | |||||||
| Metformin | 139.90 ± 14.22 df | 98.80 ± 16.14 ac | 138.60 ± 16.75 df | 224.78 ± 26.41 df | 9.10 ± 1.60 ac | 61.90 ± 16.38 df | 1.18 ± 0.27 ac |
| Test materials–WC | |||||||
| 200 mg/kg | 105.40 ± 19.47 ef | 74.80 ± 16.00 bc | 101.80 ± 19.54 df | 163.52 ± 39.41 df | 6.60 ± 2.01 c | 45.80 ± 8.90 df | 0.87 ± 0.23 c |
| 100 mg/kg | 138.60 ± 20.42 df | 96.00 ± 21.52 ac | 139.30 ± 13.74 df | 221.25 ± 30.55 df | 9.10 ± 1.91 ac | 62.30 ± 14.53 df | 1.17 ± 0.31 ac |
| 50 mg/kg | 215.70 ± 52.90 d | 151.20 ± 27.39 a | 209.30 ± 48.63 d | 383.11 ± 123.57 d | 16.00 ± 4.24 a | 107.80 ± 27.82 d | 2.07 ± 0.53 a |
| Items Groups | Liver Steatosis (%/mm2 of Hepatic Tissues) | Mean Hepatocyte Diameters (μm/cell) | Degenerative Renal Tubule Numbers (%) |
|---|---|---|---|
| Controls | |||
| NFD | 9.55 ± 4.68 | 15.93 ± 2.70 | 4.40 ± 2.91 |
| HFD | 80.77 ± 10.42 a | 42.29 ± 8.12 a | 75.70 ± 10.27 a |
| Reference | |||
| Metformin | 44.57 ± 10.20 ab | 23.61 ± 3.43 ab | 41.30 ± 10.32 ab |
| Test materials–WC | |||
| 200 mg/kg | 28.17 ± 10.74 ab | 19.97 ± 1.51 ab | 16.90 ± 5.57 ab |
| 100 mg/kg | 44.59 ± 12.01 ab | 23.82 ± 2.95 ab | 41.60 ± 11.57 ab |
| 50 mg/kg | 78.11 ± 13.04 a | 41.04 ± 8.82 a | 73.80 ± 15.27 a |
| Items Groups | Lipid Peroxidation | Antioxidant Defense System | ||
|---|---|---|---|---|
| Malondialdehyde (nM/mg Tissue) | Glutathione (μM/mg Tissue) | Catalase (U/mg Tissue) | SOD (U/mg Tissue) | |
| Controls | ||||
| NFD | 9.34 ± 1.84 | 69.63 ± 10.20 | 71.24 ± 12.12 | 7.84 ± 1.21 |
| HFD | 78.37 ± 11.04 a | 10.45 ± 1.80 a | 10.32 ± 2.61 a | 0.78 ± 0.26 a |
| Reference | ||||
| Metformin | 45.07 ± 11.48 ab | 32.31 ± 10.11 ab | 31.81 ± 10.21 ab | 2.39 ± 0.46 ab |
| Test materials–WC | ||||
| 200 mg/kg | 34.30 ± 11.03 ab | 45.49 ± 13.68 ab | 44.43 ± 10.39 ab | 3.36 ± 1.11 ab |
| 100 mg/kg | 45.99 ± 10.48 ab | 32.31 ± 13.52 ab | 31.42 ± 10.45 ab | 2.40 ± 0.58 ab |
| 50 mg/kg | 76.28 ± 16.31 a | 10.74 ± 3.21 a | 11.04 ± 3.44 a | 0.82 ± 0.40 a |
| Items Groups | Glucokinase (nM/min/mg Protein) | Glucose-6-phosphatase (nM/min/mg Protein) | PEPCK (nM/min/mg Protein) |
|---|---|---|---|
| Controls | |||
| NFD | 5.95 ± 1.19 | 110.04 ± 25.44 | 1.56 ± 0.59 |
| HFD | 1.64 ± 0.43 c | 309.59 ± 57.05 c | 5.46 ± 0.79 a |
| Reference | |||
| Metformin | 3.14 ± 0.49 cd | 173.99 ± 22.39 cd | 3.20 ± 0.77 ab |
| Test materials–WC | |||
| 200 mg/kg | 4.11 ± 0.75 cd | 145.77 ± 18.21 cd | 2.39 ± 0.60 b |
| 100 mg/kg | 3.14 ± 0.45 cd | 174.79 ± 22.81 cd | 3.20 ± 0.71 ab |
| 50 mg/kg | 1.69 ± 0.46 c | 299.60 ± 80.39 c | 5.33 ± 1.33 a |
| Items Groups | Hepatic Tissue (Relative to Control/GAPDH) | ||
|---|---|---|---|
| ACC1 | AMPKα1 | AMPKα2 | |
| Controls | |||
| NFD | 1.00 ± 0.07 | 1.01 ± 0.12 | 1.00 ± 0.07 |
| HFD | 5.31 ± 1.01 c | 0.27 ± 0.07 a | 0.25 ± 0.05 c |
| Reference | |||
| Metformin | 3.10 ± 0.77 cd | 0.48 ± 0.08 ab | 0.47 ± 0.12 cd |
| Test materials–WC | |||
| 200 mg/kg | 2.30 ± 0.46 cd | 0.60 ± 0.12 ab | 0.70 ± 0.16 cd |
| 100 mg/kg | 3.09 ± 0.40 cd | 0.48 ± 0.11 ab | 0.47 ± 0.09 cd |
| 50 mg/kg | 5.16 ± 1.02 c | 0.28 ± 0.11 a | 0.27 ± 0.10 c |
| Groups Items | Control | Reference | Test Materials—WC | |||
|---|---|---|---|---|---|---|
| NFD | HFD | Metformin | 200 mg/kg | 100 mg/kg | 50 mg/kg | |
| Adipose tissue (Relative to control/GAPDH) | ||||||
| Leptin | 1.00 ± 0.06 | 8.57 ± 1.27 c | 4.68 ± 1.13 cd | 3.44 ± 0.83 cd | 4.79 ± 1.19 cd | 8.18 ± 2.42 c |
| UCP2 | 1.01 ± 0.09 | 0.24 ± 0.05 a | 0.47 ± 0.13 ab | 0.63 ± 0.15 ab | 0.47 ± 0.13 ab | 0.24 ± 0.08 a |
| Adiponectin | 1.00 ± 0.08 | 0.15 ± 0.09 a | 0.40 ± 0.09 ab | 0.59 ± 0.14 ab | 0.41 ± 0.08 ab | 0.16 ± 0.09 a |
| C/EBPα | 1.00 ± 0.07 | 3.14 ± 0.66 c | 1.84 ± 0.24 cd | 1.33 ± 0.29 cd | 1.81 ± 0.28 cd | 3.08 ± 1.20 c |
| C/EBPβ | 1.00 ± 0.07 | 4.11 ± 0.68 c | 2.41 ± 0.51 cd | 1.84 ± 0.28 cd | 2.41 ± 0.51 cd | 4.00 ± 0.93 c |
| SREBP1 c | 1.00 ± 0.06 | 2.97 ± 0.86 c | 1.73 ± 0.20 cd | 1.27 ± 0.15 cd | 1.73 ± 0.29 cd | 2.90 ± 0.99 c |
| PPARα | 1.00 ± 0.09 | 0.16 ± 0.05 c | 0.32 ± 0.06 cd | 0.50 ± 0.18 cd | 0.31 ± 0.08 cd | 0.17 ± 0.06 c |
| PPARγ | 1.01 ± 0.10 | 8.09 ± 0.91 c | 4.63 ± 0.97 cd | 3.61 ± 1.07 cd | 4.68 ± 1.22 cd | 7.82 ± 1.38 c |
| FAS | 1.00 ± 0.05 | 15.40 ± 3.36 c | 8.86 ± 0.63 cd | 6.91 ± 1.85 cd | 8.85 ± 0.85 cd | 15.06 ± 4.30 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.-G.; Bashir, K.M.I.; Kim, K.-Y.; Shin, S.; Choi, M.-W.; Hong, E.-J.; Choi, S.-H.; Kim, J.-W.; Choi, J.-S.; Ku, S.-K. Evaluation of Dose-Dependent Obesity and Diabetes-Related Complications of Water Chestnut (Fruit of Trapa japonica) Extracts in Type II Obese Diabetic Mice Induced by 45% Kcal High-Fat Diet. Medicina 2022, 58, 189. https://doi.org/10.3390/medicina58020189
Kang H-G, Bashir KMI, Kim K-Y, Shin S, Choi M-W, Hong E-J, Choi S-H, Kim J-W, Choi J-S, Ku S-K. Evaluation of Dose-Dependent Obesity and Diabetes-Related Complications of Water Chestnut (Fruit of Trapa japonica) Extracts in Type II Obese Diabetic Mice Induced by 45% Kcal High-Fat Diet. Medicina. 2022; 58(2):189. https://doi.org/10.3390/medicina58020189
Chicago/Turabian StyleKang, Hyun-Gu, Khawaja Muhammad Imran Bashir, Ki-Young Kim, Su Shin, Min-Woo Choi, Eun-Jin Hong, Seong-Hun Choi, Joo-Wan Kim, Jae-Suk Choi, and Sae-Kwang Ku. 2022. "Evaluation of Dose-Dependent Obesity and Diabetes-Related Complications of Water Chestnut (Fruit of Trapa japonica) Extracts in Type II Obese Diabetic Mice Induced by 45% Kcal High-Fat Diet" Medicina 58, no. 2: 189. https://doi.org/10.3390/medicina58020189
APA StyleKang, H. -G., Bashir, K. M. I., Kim, K. -Y., Shin, S., Choi, M. -W., Hong, E. -J., Choi, S. -H., Kim, J. -W., Choi, J. -S., & Ku, S. -K. (2022). Evaluation of Dose-Dependent Obesity and Diabetes-Related Complications of Water Chestnut (Fruit of Trapa japonica) Extracts in Type II Obese Diabetic Mice Induced by 45% Kcal High-Fat Diet. Medicina, 58(2), 189. https://doi.org/10.3390/medicina58020189







