Vitamin D Implications and Effect of Supplementation in Endocrine Disorders: Autoimmune Thyroid Disorders (Hashimoto’s Disease and Grave’s Disease), Diabetes Mellitus and Obesity
Abstract
:1. Introduction
2. Material and Methods
3. Vitamin D General Implications in Health and Daily Requirements
4. Role of Vitamin D in Thyroid Autoimmune Diseases and Effects of Supplementation
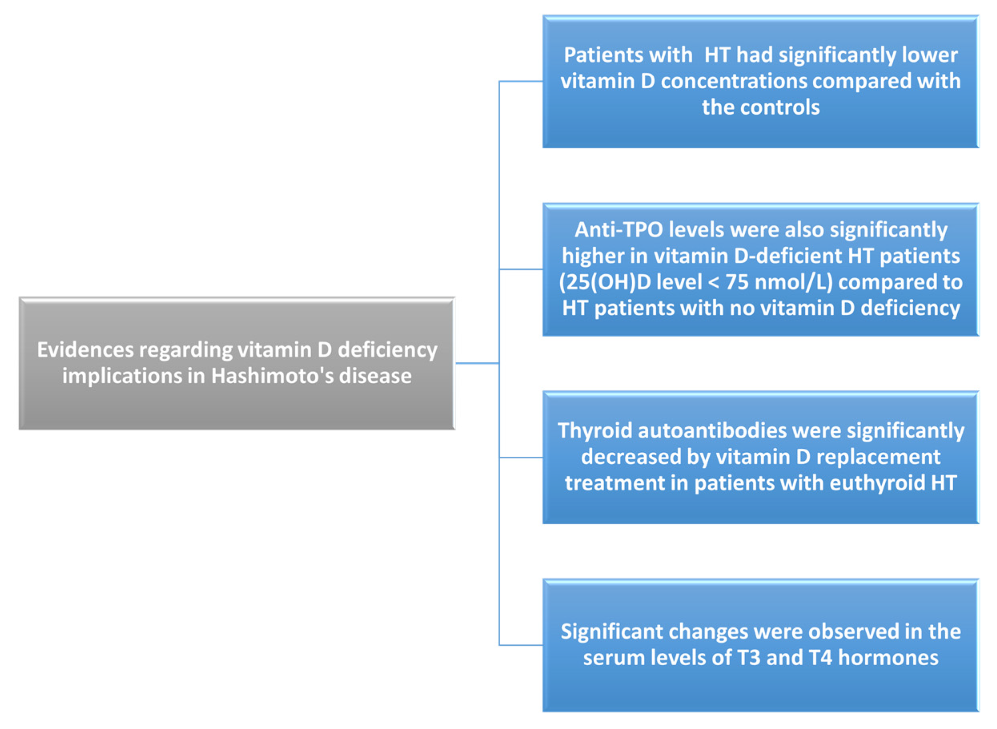
4.1. Implications in Hashimoto’s Disease
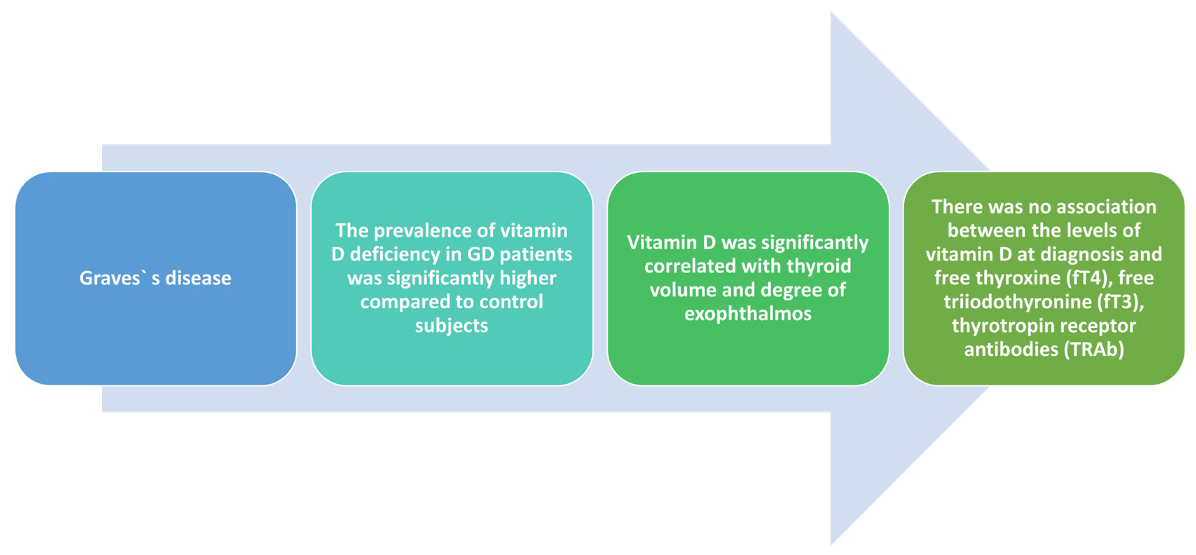
4.2. Implications of Vitamin D Deficiency in Graves’ Disease
5. Vitamin D Deficit in Type 2 Diabetes Mellitus and Effects of Supplementation
6. Deficit in Obesity and Impact of Supplementation on Weight Loss
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. 2017, 18, 335–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscogiuri, G.; Mitri, J.; Mathieu, C.; Badenhoop, K.; Tamer, G.; Orio, F.; Mezza, T.; Vieth, R.; Colao, A.; Pittas, A. Mechanisms in endocrinology: Vitamin D as a potential contributor in endocrine health and disease. Eur. J. Endocrinol. 2014, 171, R101–R110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zynat, J.; Guo, Y.; Osiman, R.; Tuhuti, A.; Zhao, H.; Abdunaimu, M.; Wang, H.; Jin, X.; Xing, S. Low serum vitamin D is associated with anti-thyroid-globulin antibody in female individuals. Int. J. Endocrinol. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymczak-Pajor, I.; Śliwińska, A. Analysis of association between Vitamin D deficiency and insulin resistance. Nutrients 2019, 11, 794. [Google Scholar] [CrossRef] [Green Version]
- Adenote, A.; Dumic, I.; Madrid, C.; Barusya, C.; Nordstrom, C.W.; Rueda Prada, L. NAFLD and Infection, a Nuanced Relationship. Can. J. Gastroenterol. Hepatol. 2021, 2021, 5556354. [Google Scholar] [CrossRef]
- Hossein-Nezhad, A.; Spira, A.; Holick, M.F. Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: A randomized double-blind clinical trial. PLoS ONE 2013, 8, e58725. [Google Scholar] [CrossRef] [Green Version]
- Pike, J.W.; Meyer, M.B. Fundamentals of vitamin D hormone-regulated gene expression. J. Steroid Biochem. Mol. Biol. 2014, 144, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [Green Version]
- Heaney, R.P. Serum 25-hydroxyvitamin D is a reliable indicator of vitamin D status. Am. J. Clin. Nutr. 2011, 94, 619–620. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D status: Measurement, interpretation, and clinical application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M. Consensus statement from 2nd International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 2, 89–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollis, B.W.; Wagner, C.L. Clinical review: The role of the parent compound vitamin D with respect to metabolism and function: Why clinical dose intervals can affect clinical outcomes. J. Clin. Endocrinol. Metab. 2013, 98, 4619–4628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzoli, R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021, 33, 19–24. [Google Scholar] [CrossRef]
- Kust, D.; Matesa, N. The impact of familial predisposition on the development of Hashimoto’s thyroiditis. Acta Clin. Belg. 2020, 75, 104–108. [Google Scholar] [CrossRef]
- Moon, S.; Chung, H.S.; Yu, J.M.; Yoo, H.J.; Park, J.H.; Kim, D.S.; Park, Y.J. Associations between Hashimoto Thyroiditis and clinical outcomes of papillary thyroid cancer: A meta-analysis of observational studies. Endocrinol. Metab. 2018, 33, 473–484. [Google Scholar] [CrossRef]
- Cai, Y.J.; Wang, F.; Chen, Z.X.; Li, L.; Fan, H.; Wu, Z.B.; Ge, J.F.; Hu, W.; Wang, Q.N.; Zhu, D.F. Hashimoto’s thyroiditis induces neuroinflammation and emotional alterations in euthyroid mice. J. Neuroinflammation 2018, 15, 299. [Google Scholar] [CrossRef] [Green Version]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The effect of vitamin D on thyroid autoimmunity in levothyroxine-treated women with Hashimoto’s thyroiditis and normal vitamin D status. Exp. Clin. Endocrinol. Diabetes 2017, 125, 229–233. [Google Scholar] [CrossRef]
- Lang, P.O.; Aspinall, R. Can we translate vitamin D immunomodulating effect on innate and adaptive immunity to vaccine response? Nutrients 2015, 7, 2044–2060. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, K.; Wessner, B.; Laggner, U.; Ploder, M.; Tamandl, D.; Friedl, J.; Zügel, U.; Steinmeyer, A.; Pollak, A.; Roth, E. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Our. J. Immunol. 2006, 36, 361–370. [Google Scholar] [CrossRef]
- Kundu, R.; Theodoraki, A.; Haas, C.T.; Zhang, Y.; Chain, B.; Kriston-Vizi, J.; Noursadeghi, M.; Khoo, B. Cell-type-specific modulation of innate immune signalling by vitamin D in human mononuclear phagocytes. Immunology 2017, 150, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, G.; Zhu, Y.; Fang, L. Correlation between Hashimoto’s thyroiditis-related thyroid hormone levels and 25-hydroxyvitamin D. Front. Endocrinol. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ucan, B.; Sahin, M.; Sayki Arslan, M.; Colak Bozkurt, N.; Kizilgul, M.; Güngünes, A.; Cakal, E.; Ozbek, M. Vitamin D treatment in patients with Hashimoto’s thyroiditis may decrease the development of hypothyroidism. Int. J. Vitam. Nutr. Res. 2016, 86, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Gee, E.; Halloran, B.; Kowalski, M.A.; Ryzen, E.; Haddad, J.G. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J. Clin. Endocrinol. Metab. 1986, 63, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.L. Vitamin D hydroxylases. J. Cell. Biochem. 1992, 49, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Pani, M.A.; Knapp, M.; Donner, H.; Braun, J.; Baur, M.P.; Usadel, K.H.; Badenhoop, K. Vitamin D receptor allele combinations influence genetic susceptibility to type 1 diabetes in Germans. Diabetes 2000, 49, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Horst-Sikorska, W.; Ignaszak-Szczepaniak, M.; Marcinkowska, M.; Kaczmarek, M.; Stajgis, M.; Slomski, R. Association analysis of vitamin D receptor gene polymorphisms with bone mineral density in young women with Graves’ disease. Acta Biochim. Pol. 2008, 55, 371–380. [Google Scholar] [CrossRef]
- Kurylowicz, A.; Ramos-Lopez, E.; Bednarczuk, T.; Badenhoop, K. Vitamin D-Binding Protein (DBP) Gene polymorphism is associated with Graves’ disease and the vitamin D status in a Polish population study. Exp. Clin. Endocrinol. Diabetes 2006, 114, 329–335. [Google Scholar] [CrossRef]
- Ban, Y.; Ban, Y.; Taniyama, M.; Katagiri, T. Vitamin D receptor initiation codon polymorphism in Japanese patients with Graves’ disease. Thyroid 2000, 10, 475–480. [Google Scholar] [CrossRef]
- Miller, A.E.; Morgante, L.A.; Buchwald, L.Y.; Nutile, S.M.; Coyle, P.K.; Krupp, L.B.; Doscher, C.A.; Lublin, F.D.; Knobler, R.L.; Trantas, F.; et al. A Multicenter, randomized, double-blind, placebo-controlled trial of Influenza immunization in multiple sclerosis. Neurology 1997, 48, 312–314. [Google Scholar] [CrossRef]
- Kivity, S.; Agmon-Levin, N.; Zisappl, M.; Shapira, Y.; Nagy, E.V.; Dankó, K.; Szekanecz, Z.; Langevitz, P.; Shoenfeld, Y. Vitamin D and autoimmune thyroid diseases. Cell. Mol. Immunol. 2011, 8, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazokopakis, E.E.; Papadomanolaki, M.G.; Tsekouras, K.C.; Evangelopoulos, A.D.; Kotsiris, D.A.; Tzortzinis, A.A. Is vitamin D related to pathogenesis and treatment of Hashimoto’s thyroiditis? Hell. J. Nucl. Med. 2015, 18, 222–227. [Google Scholar] [PubMed]
- Chaudhary, S.; Dutta, D.; Kumar, M.; Saha, S.; Mondal, S.A.; Kumar, A.; Mukhopadhyay, S. Vitamin D supplementation reduces thyroid peroxidase antibody levels in patients with autoimmune thyroid disease: An open-labeled randomized controlled trial. Indian J. Endocrinol. Metab. 2016, 20, 391–398. [Google Scholar] [PubMed]
- Chahardoli, R.; Saboor-Yaraghi, A.-A.; Amouzegar, A.; Khalili, D.; Vakili, A.Z.; Azizi, F. Can supplementation with vitamin D modify thyroid autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and thyroid profile (T3, T4, TSH) in Hashimoto’s Thyroiditis? A Double Blind, Randomized Clinical Trial. Horm. Metab. Res. 2019, 51, 296–301. [Google Scholar] [CrossRef]
- Behera, K.K.; Saharia, G.K.; Hota, D.; Sahoo, D.P.; Sethy, M.; Srinivasan, A. Effect of Vitamin D supplementation on thyroid autoimmunity among subjects of autoimmune thyroid disease in a coastal province of India: A randomized open-label trial. Niger. Med. J. 2020, 61, 237–240. [Google Scholar] [CrossRef]
- Štefanić, M.; Tokić, S. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto’s thyroiditis: A systematic review, meta-analysis and meta-regression of observational studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and meta-analysis of observational studies. BMC Endocr Disord. 2021, 21, 171. [Google Scholar] [CrossRef]
- Yasuda, T.; Okamoto, Y.; Hamada, N.; Miyashita, K.; Takahara, M.; Sakamoto, F.; Miyatsuka, T.; Kitamura, T.; Katakami, N.; Kawamori, D.; et al. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine 2013, 43, 230–232. [Google Scholar] [CrossRef] [Green Version]
- Ghitea, T.C.; Aleya, L.; Tit, D.M.; Behl, T.; Stoicescu, M.; Sava, C.; Iovan, C.; El-Kharoubi, A.; Uivarosan, D.; Pallag, A.; et al. Influence of diet and sport on the risk of sleep apnea in patients with metabolic syndrome associated with hypothyroidism—A 4-year survey. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Rotondi, M.; Chiovato, L. The chemokine system as a therapeutic target in autoimmune thyroid diseases: A focus on the interferon-γ inducible chemokines and their receptor. Curr. Pharm. Des. 2011, 17, 3202–3216. [Google Scholar] [CrossRef]
- Sheriba, N.; Elewa, A.A.; Mahdy, M.; Bahaa El Din, A.; Ibrahim, N.; Marawan, D.; Abd El Moneim, T. Effect of vitamin D3 in treating hyperthyroidism in patients with Graves’ disease. Egypt. J. Intern. Med. 2017, 29, 64. [Google Scholar] [CrossRef]
- Planck, T.; Shahida, B.; Malm, J.; Manjer, J. Vitamin D in Graves’ disease: Levels, correlation with laboratory and clinical parameters, and genetics. Eur. Thyroid J. 2018, 7, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.Y.; Cao, B.; Yin, J.; Wang, D.F.; Chen, K.L.; Lu, Q.B. Vitamin D and Graves’ disease: A meta-analysis update. Nutrients 2015, 7, 3813–3827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasuda, T.; Okamoto, Y.; Hamada, N.; Miyashita, K.; Takahara, M.; Sakamoto, F.; Miyatsuka, T.; Kitamura, T.; Katakami, N.; Kawamori, D.; et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves’ disease. Endocrine 2012, 42, 739–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Garach, A.; García-Fontana, B.; Muñoz-Torres, M. Vitamin D status, calcium intake and risk of developing type 2 diabetes: An unresolved issue review. Nutrients 2019, 11, 642. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, D.S.; Kang, S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing PPAR-γ expression in nonobese Type 2 diabetic rats. J. Nutr. Biochem. 2016, 27, 257–265. [Google Scholar] [CrossRef]
- Gonçalves de Carvalho, C.M.; Ribeiro, S.M. Aging, low-grade systemic inflammation and vitamin D: A mini review. Eur. J. Clin. Nutr. 2017, 71, 434–440. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Bucci, C.; Lo Monaco, M.R.; Bentivoglio, A.R.; Coelho-Júnior, H.J.; Landi, F.; Bernabei, R.; Marzetti, E. Mitochondrial dysfunction and aging: Insights from the analysis of extracellular vesicles. Int. J. Mol. Sci. 2019, 20, 805. [Google Scholar] [CrossRef] [Green Version]
- Mousa, A.; Naderpoor, N.; Teede, H.; Scragg, R.; de Courten, B. Vitamin D supplementation for improvement of chronic low-grade inflammation in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2018, 76, 380–394. [Google Scholar] [CrossRef] [Green Version]
- Dunlop, T.W.; Väisänen, S.; Frank, C.; Molnár, F.; Sinkkonen, L.; Carlberg, C. The human peroxisome proliferator-activated receptor delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J. Mol. Biol. 2005, 349, 248–260. [Google Scholar] [CrossRef]
- Kang, S.; Tsai, L.T.; Zhou, Y.; Evertts, A.; Xu, S.; Griffin, M.J.; Issner, R.; Whitton, H.J.; Garcia, B.A.; Epstein, C.B.; et al. Identification of nuclear hormone receptor pathways causing insulin resistance by transcriptional and epigenomic analysis. Nat. Cell Biol. 2015, 17, 44–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, T.J.; Skeaff, C.M.; Rockell, J.E.P.; Venn, B.J.; Lambert, A.; Todd, J.; Khor, G.L.; Loh, S.P.; Muslimatun, S.; Agustina, R.; et al. Vitamin D status and its association with parathyroid hormone concentrations in women of child-bearing age living in Jakarta and Kuala Lumpur. Eur. J. Clin. Nutr. 2008, 62, 373–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alkharfy, K.M.; Al-Daghri, N.M.; Yakout, S.M.; Hussain, T.; Mohammed, A.K.; Krishnaswamy, S. Influence of vitamin D treatment on transcriptional regulation of insulin-sensitive genes. Metab. Syndr. Relat. Disord. 2013, 11, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Krul-Poel, Y.H.M.; Ter Wee, M.M.; Lips, P.; Simsek, S. Management of endocrine disease: The effect of vitamin D supplementation on glycaemic control in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Eur. J. Endocrinol. 2017, 176, R1–R14. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Liu, Y.; Zheng, Y.; Wang, P.; Zhang, Y. The effect of vitamin D supplementation on glycemic control in type 2 diabetes patients: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 375. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Jin’an Chen, X.S.; Wang, L.; Wang, A. Efficacy of vitamin D supplementation on glycemic control in type 2 diabetes patients. Medicine 2019, 98, e14970. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.; Tang, J.; Li, J.; Chong, W.; Hai, Y.; Feng, Y.; Lunsford, L.D.; Xu, P.; Jia, D.; et al. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: A systematic review and meta-analysis. Diabetes Care 2020, 43, 1650–1658. [Google Scholar] [CrossRef]
- Martini, L.A.; Wood, R.J. Vitamin D status and the metabolic syndrome. Nutr. Rev. 2006, 64, 479–486. [Google Scholar] [CrossRef]
- Blumberg, J.M.; Tzameli, I.; Astapova, I.; Lam, F.S.; Flier, J.S.; Hollenberg, A.N. Complex Role of the Vitamin D Receptor and Its Ligand in Adipogenesis in 3T3-L1 Cells. J. Boil. Chem. 2006, 281, 11205–11213. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Bae, S.; Yoon, Y. Anti-adipogenic effects of 1,25-dihydroxyvitamin D3 are mediated by the maintenance of the wingless-type MMTV integration site/beta-catenin pathway. Int. J. Mol. Med. 2012, 30, 1219–1224. [Google Scholar] [CrossRef] [Green Version]
- Leblanc, E.S.; Rizzo, J.H.; Pedula, K.L.; Ensrud, K.E.; Cauley, J.; Hochberg, M.; Hillier, T.A. Associations between 25-hydroxyvitamin D and weight gain in elderly women. J. Women’s Health 2012, 21, 1066–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mai, X.M.; Chen, Y.; Camargo, C.A., Jr. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in adults: The HUNT study. Am. J. Epidemiol. 2012, 175, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 393–404. [Google Scholar] [CrossRef]
- Vimaleswaran, K.S.; Berry, D.J.; Lu, C.; Tikkanen, E.; Pilz, S.; Hiraki, L.T.; Cooper, J.D.; Dastani, Z.; Li, R.; Houston, D.K.; et al. Causal relationship between obesity and vitamin D status: Bidirectional mendelian randomization analysis of multiple cohorts. PLoS Med. 2013, 10, e1001383. [Google Scholar] [CrossRef]
- Shanmugalingam, T.; Crawley, D.; Bosco, C.; Melvin, J.; Rohrmann, S.; Chowdhury, S.; Holmberg, L.; Van Hemelrijck, M. Obesity and cancer: The role of vitamin D. BMC Cancer 2014, 14, 712. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and metaanalysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Soares, M.J.; Calton, E.K.; Zhao, Y.; Hallett, J. Vitamin D supplementation and body weight status: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2014, 15, 528–537. [Google Scholar] [CrossRef]
- Chandler, P.D.; Wang, L.; Zhang, X.; Sesso, H.D.; Moorthy, M.V.; Obi, O.; Lewis, J.; Prince, R.L.; Danik, J.S.; Manson, J.E.; et al. Effect of vitamin D supplementation alone or with calcium on adiposity measures: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2015, 73, 577–593. [Google Scholar] [CrossRef]
- Manousopoulou, A.; Al-Daghri, N.M.; Garbis, S.D.; Chrousos, G.P. Vitamin D and cardiovascular risk among adults with obesity: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2015, 45, 1113–1126. [Google Scholar] [CrossRef] [Green Version]
- Karampela, I.; Sakelliou, A.; Vallianou, N.; Christodoulatos, G.S.; Magkos, F.; Dalamaga, M. Vitamin D and obesity: Current evidence and controversies. Curr. Obes. Rep. 2021, 10, 162–180. [Google Scholar] [CrossRef]

| Identification of Studies via Databases and Registers for the Realization of the Review | ||
|---|---|---|
| Identification | Key words with “AND” operator | “autoimmune thyroid disorders” AND “vitamin D”, “Hashimoto’s diseases” AND “vitamin D”, “Graves’ disease” AND “vitamin D”, “type 2 diabetes mellitus” AND “vitamin D”, “obesity” AND “vitamin D”, etc. |
| Consulted databases | Web of Science, PubMed | |
| Criteria for inclusion | Review or original article, relevant for the topic and thematic | |
| Criteria for exclusion | Abstract paper, articles where full text was not available, not relevant to the topic or thematic | |
| Records identified | 185 records | |
| Articles after duplicate removal (duplicates = 21) | 164 records | |
| Full-text analysis | Appliance of inclusion and exclusion criteria (eliminated 49 articles) | 121 records |
| Final bibliographical sources | Verification and agreement on article relevance and quality | 72 records |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galușca, D.; Popoviciu, M.S.; Babeș, E.E.; Vidican, M.; Zaha, A.A.; Babeș, V.V.; Jurca, A.D.; Zaha, D.C.; Bodog, F. Vitamin D Implications and Effect of Supplementation in Endocrine Disorders: Autoimmune Thyroid Disorders (Hashimoto’s Disease and Grave’s Disease), Diabetes Mellitus and Obesity. Medicina 2022, 58, 194. https://doi.org/10.3390/medicina58020194
Galușca D, Popoviciu MS, Babeș EE, Vidican M, Zaha AA, Babeș VV, Jurca AD, Zaha DC, Bodog F. Vitamin D Implications and Effect of Supplementation in Endocrine Disorders: Autoimmune Thyroid Disorders (Hashimoto’s Disease and Grave’s Disease), Diabetes Mellitus and Obesity. Medicina. 2022; 58(2):194. https://doi.org/10.3390/medicina58020194
Chicago/Turabian StyleGalușca, Dorina, Mihaela Simona Popoviciu, Emilia Elena Babeș, Mădălina Vidican, Andreea Atena Zaha, Vlad Victor Babeș, Alexandru Daniel Jurca, Dana Carmen Zaha, and Florian Bodog. 2022. "Vitamin D Implications and Effect of Supplementation in Endocrine Disorders: Autoimmune Thyroid Disorders (Hashimoto’s Disease and Grave’s Disease), Diabetes Mellitus and Obesity" Medicina 58, no. 2: 194. https://doi.org/10.3390/medicina58020194







