Soft Tissue Sarcomas: A 16-Year Experience of a Tertiary Referral Hospital in North Jordan
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SEER*Stat Database: Incidence—SEER 9 Regs Research Data, November 2010 Sub (1973–2008) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.S., 1969–2009 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2011, based on the November 2010 submission. Surveillance, Epidemiology, and End Results (SEER) Program. Available online: http://www.seer.cancer.gov (accessed on 1 November 2021).
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma epidemiology and etiology: Potential environmental and genetic factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumors, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The epidemiology of sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [Green Version]
- Penel, N.; Grosjean, J.; Robin, Y.M.; Vanseymortier, L.; Clisant, S.; Adenis, A. Frequency of certain established risk factors in soft tissue sarcomas in adults: A prospective descriptive study of 658 cases. Sarcoma 2008, 2008, 459386. [Google Scholar] [CrossRef]
- Fabiano, S.; Contiero, P.; Barigelletti, G.; D’Agostino, A.; Tittarelli, A.; Mangone, L.; Bisceglia, I.; Bongiorno, S.; De Lorenzis, L.E.; Mazzoleni, G.; et al. Epidemiology of Soft Tissue Sarcoma and Bone Sarcoma in Italy: Analysis of Data from 15 Population-Based Cancer Registries. Sarcoma 2020, 2020, 6142613. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Montesco, M.C.; Coindre, J.M.; Dei Tos, A.P.; Lurkin, A.; Ranchère-Vince, D.; Vecchiato, A.; Decouvelaere, A.V.; Mathoulin-Pélissier, S.; Albert, S.; et al. Sarcoma: Concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann. Oncol. 2012, 23, 2442–2449. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures 2021; American Cancer Society: Atlanta, GA, USA, 2021; Available online: https://www.cancer.org/cancer/soft-tissue-sarcoma/about/key-statistics.html#references (accessed on 1 November 2021).
- Saltus, C.W.; Calingaert, B.; Candrilli, S.; Lorenzo, M.; D’yachkova, Y.; Otto, T.; Wagner, U.; Kaye, J.A. Epidemiology of Adult Soft-Tissue Sarcomas in Germany. Sarcoma 2018, 2018, 5671926. [Google Scholar] [CrossRef]
- Dugandzija, T.; Mikov, M.M.; Solajic, N.; Nikolin, B.; Trifunovic, J.; Ilic, M. Increasing frequency of soft tissue sarcomas in Vojvodina—comparison with the literature. Asian Pac. J. Cancer Prev. 2014, 15, 1011–1014. [Google Scholar] [CrossRef] [Green Version]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. (Eds.) International Classification of Diseases for Oncology (ICD-O), 3rd ed.; World Health Organization: Geneva, Switzerland, 2013; Available online: https://apps.who.int/iris/bitstream/handle/10665/96612/9789241548496_eng.pdf (accessed on 1 November 2021).
- Gupta, A.; Rao, H.K.; Gupta, S. The incidence of soft tissue sarcoma in Dakshina Kannada: Study in a District Government Hospital. Indian J. Surg. 2009, 71, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Gaynor, J.J.; Tan, C.C.; Casper, E.S.; Collin, C.F.; Friedrich, C.; Shiu, M.; Hajdu, S.I.; Brennan, M.F. Refinement of clinicopathologic staging for localized soft tissue sarcoma of the extremity: A study of 423 adults. J. Clin. Oncol. 1992, 10, 1317–1329. [Google Scholar] [CrossRef]
- Eilber, F.C.; Brennan, M.F.; Eilber, F.R.; Dry, S.M.; Singer, S.; Kattan, M.W. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer 2004, 101, 2270–2275. [Google Scholar] [CrossRef]
- Coindre, J.M. Grading of soft tissue sarcomas: Review and update. Arch. Pathol. Lab. Med. 2006, 130, 1448–1453. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Guillou, L.; Coindre, J.M.; Bonichon, F.; Nguyen, B.B.; Terrier, P.; Collin, F.; Vilain, M.O.; Mandard, A.M.; Le Doussal, V.; Leroux, A.; et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J. Clin. Oncol. 1997, 15, 350–362. [Google Scholar] [CrossRef]
- Bashaireh, K.M.; Alorjani, M.; Jahmani, R.A.; Al Khateeb, A.; Nimri, F.; Al-Ebbini, M.A.; Ababneh, A.R.M. Primary Bone Tumors in North of Jordan. J. Epidemiol. Glob. Health 2021, 11, 132–136. [Google Scholar] [CrossRef]
- Population—Jordan Department of Statistics. Available online: http://dosweb.dos.gov.jo (accessed on 14 February 2021).
- Mustafa, M.; Al-jarrah, O.; Hamoury, M.K.; Alhiwat, S.; Al-Hassanat, O. Pediatric rhabdomyosarcoma: A 7-year experience at King Hussein Medical Center. J. R. Med. Serv. 2016, 23, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Yaser, S.; Salah, S.; Al-Shatti, M.; Abu-Sheikha, A.; Shehadeh, A.; Sultan, I.; Salem, A.; Sughayer, M.; Al-Loh, S.; Al-Mousa, A. Prognostic factors that govern localized synovial sarcoma: A single institution retrospective study on 51 patients. Med. Oncol. 2014, 31, 958. [Google Scholar] [CrossRef]
- Amr, S.S.; Farah, G.R.; Muhtaseb, H.H.; Al-Hajj, H.A.; Levene, A. Clear cell sarcoma: Report of two cases with ultrastructural observations and review of the literature. Clin. Oncol. 1984, 10, 59–65. [Google Scholar]
- Abou Chaar, M.K.; Jaber, O.I.; Asha, W.; Abdel Al, S. Novel Double Central Ray Amputation of the Third and Fourth Digits: Case Report and Literature Review. Case Rep. Oncol. 2020, 13, 91–99. [Google Scholar] [CrossRef]
- Amr, S.S.; Shihabi, N.K.; Al Hajj, H. Synovial sarcoma of the esophagus. Am. J. Otolaryngol. 1984, 5, 266–269. [Google Scholar] [CrossRef]
- Salah, S.; Al-Ibraheem, A.; Daboor, A.; Al-Hussaini, M. Synovial sarcoma presenting with huge mediastinal mass: A case report and review of literature. BMC Res. Notes 2013, 6, 240. [Google Scholar] [CrossRef] [Green Version]
- Muhsen, B.A.; Ghzawi, A.; Fares, A.S.; Al-Hussaini, M.; Salah, S. Metastatic myxoid liposarcoma of the brain: A case report and review of the literature. Future Sci. OA 2021, 7, FSO756. [Google Scholar] [CrossRef]
- Abdel Al, S.; Abou Chaar, M.K.; Asha, W.; Al-Najjar, H.; Al-Hussaini, M. Fungating malignant peripheral nerve sheath tumor arising from a slow-growing mass in the forearm: A case report and review of the literature. J. Med. Case Rep. 2020, 14, 91. [Google Scholar] [CrossRef]
- Ross, J.A.; Severson, R.K.; Davis, S.; Brooks, J.J. Trends in the incidence of soft tissue sarcomas in the United States from 1973 through 1987. Cancer 1993, 72, 486–490. [Google Scholar] [CrossRef]
- Gustafson, P. Soft tissue sarcoma. Epidemiology and prognosis in 508 patients. Acta Orthop. Scand. Suppl. 1994, 65, 2–31. [Google Scholar] [CrossRef]
- Storm, H.H. Cancers of the soft tissues. Cancer Surv. 1994, 19–20, 197–217. [Google Scholar]
- Levi, F.; La Vecchia, C.; Randimbison, L.; Te, V.C. Descriptive epidemiology of soft tissue sarcomas in Vaud, Switzerland. Eur. J. Cancer 1999, 35, 1711–1716. [Google Scholar] [CrossRef]
- Toro, J.R.; Travis, L.B.; Wu, H.J.; Zhu, K.; Fletcher, C.D.; Devesa, S.S. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. Int. J. Cancer 2006, 119, 2922–2930. [Google Scholar] [CrossRef]
- Wibmer, C.; Leithner, A.; Zielonke, N.; Sperl, M.; Windhager, R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann. Oncol. 2010, 21, 1106–1111. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, R.; Zhang, S.; Zeng, H.; Li, H.; Chen, W. Incidence, distribution of histological subtypes and primary sites of soft tissue sarcoma in China. Cancer Biol. Med. 2019, 16, 565–574. [Google Scholar] [CrossRef]
- Rare Cancer Network in Europe. Available online: http://www.rarecarenet.eu (accessed on 14 February 2021).
- National Cancer Institute, Surveillance Research Program, National Cancer Institute, Bethesda, MA, USA, 2016. Available online: https://seer.cancer.gov/statfacts/html/soft.html (accessed on 1 November 2021).
- Schuurman, B.; Meyer, S.; Cuesta, M.A.; Nauta, J.J. Stijgende frequentie van weke-delensarcomen in Nederland [Increasing frequency of soft tissue sarcomas in The Netherlands]. Ned. Tijdschr. Geneeskd. 1992, 136, 1556–1560. (In Dutch) [Google Scholar]
- Bhurgri, Y.; Bhurgri, H.; Pervez, S.; Kayani, N.; Usman, A.; Bashir, I.; Bhurgri, A.; Hasan, S.H.; Zaidi, S.M. Epidemiology of soft tissue sarcomas in Karachi South, Pakistan (1995–1997). Asian Pac. J. Cancer Prev. 2008, 9, 709–714. [Google Scholar]
- Mastrangelo, G.; Coindre, J.M.; Ducimetière, F.; Dei Tos, A.P.; Fadda, E.; Blay, J.Y.; Buja, A.; Fedeli, U.; Cegolon, L.; Frasson, A.; et al. Incidence of soft tissue sarcoma and beyond: A population-based prospective study in 3 European regions. Cancer 2012, 118, 5339–5348. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Sultan, I.; Huang, T.T.; Rodriguez-Galindo, C.; Shehadeh, A.; Meazza, C.; Ness, K.K.; Casanova, M.; Spunt, S.L. Soft tissue sarcoma across the age spectrum: A population-based study from the Surveillance Epidemiology and End Results database. Pediatr. Blood Cancer 2011, 57, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Ogura, K.; Higashi, T.; Kawai, A. Statistics of soft-tissue sarcoma in Japan: Report from the Bone and Soft Tissue Tumor Registry in Japan. J. Orthop. Sci. 2017, 22, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Statistics Bureau of Japan. 2021. Available online: https://www.stat.go.jp (accessed on 14 February 2021).
- The World Bank. Available online: https://data.worldbank.org/indicator/SP.DYN.LE00.IN?locations=JO (accessed on 20 December 2021).
- Tsujimoto, M.; Aozasa, K.; Ueda, T.; Sakurai, M.; Ishiguro, S.; Kurata, A.; Ono, K.; Matsumoto, K. Soft tissue sarcomas in Osaka, Japan (1962–1985): Review of 290 cases. Jpn. J. Clin. Oncol. 1988, 18, 231–234. [Google Scholar] [PubMed]
- Nijhuis, P.H.; Schaapveld, M.; Otter, R.; Molenaar, W.M.; van der Graaf, W.T.; Hoekstra, H.J. Epidemiological aspects of soft tissue sarcomas (STS)—consequences for the design of clinical STS trials. Eur. J. Cancer 1999, 35, 1705–1710. [Google Scholar] [CrossRef]
- Hui, J.Y. Epidemiology and Etiology of Sarcomas. Surg. Clin. N. Am. 2016, 96, 901–914. [Google Scholar] [CrossRef]


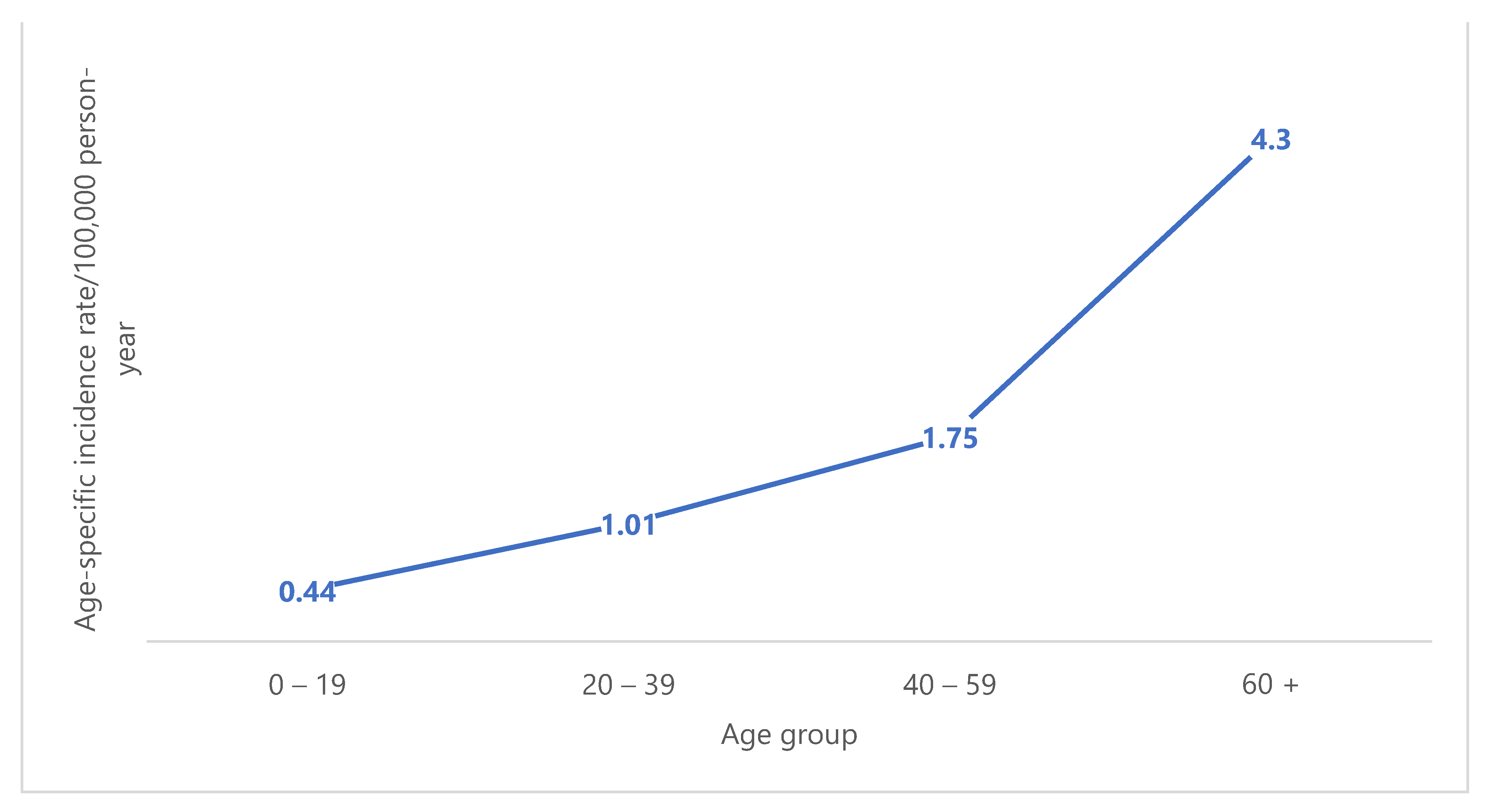

| Histological Type of Sarcoma | n | IR | 95% CI |
|---|---|---|---|
| Liposarcoma | 30 | 0.1984 | 0.14–0.28 |
| Rhabdomyosarcoma | 27 | 0.1786 | 0.12–0.26 |
| Leiomyosarcoma | 16 | 0.1058 | 0.06–0.17 |
| MPNST | 14 | 0.0926 | 0.05–0.16 |
| Gender | Relative Frequency | LPS | RMS | LMS | MPNST | DFSP | Ewing Sarcoma/PNET | SS | Others | |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | 0–19 y | 23.6 | 61.9 | 4.8 | 4.8 | 19 | 9.5 | |||
| 20–39 y | 29.2 | 19.2 | 7.7 | 7.7 | 7.7 | 15.4 | 3.8 | 23.1 | 15.4 | |
| 40–59 y | 21.4 | 21 | 15.8 | 26.3 | 5.3 | 5.3 | 5.3 | 21 | ||
| 60+ y | 25.8 | 26 | 8.7 | 4.4 | 4.4 | 4.4 | 52.2 | |||
| Female | 0–19 y | 16.2 | 63.6 | 9.1 | 9.1 | 9.1 | 9.1 | |||
| 20–39 y | 33.8 | 21.8 | 8.7 | 17.4 | 8.7 | 13 | 13 | 17.4 | ||
| 40–59 y | 26.5 | 33.3 | 11.1 | 5.6 | 11.1 | 5.6 | 5.6 | 27.8 | ||
| 60+ y | 23.5 | 25 | 6.25 | 37.5 | 31.25 |
| ICD-O-3 Topography Code | Location | n (%) |
|---|---|---|
| C15-C26 | Digestive organs | 5 (3.18) |
| C30-C39 | Respiratory system and intrathoracic organs | 7 (4.46) |
| C42 | Hematopoietic and reticuloendothelial system | 1 (0.64) |
| C44 | Skin | 16 (10.2) |
| C47 | Peripheral nerves and autonomic nervous system | 14 (8.9) |
| C48 | retroperitoneum and peritoneum | 16 (10.2) |
| C49 | Connective, subcutaneous and other soft tissues | 91 (58) |
| C50 | Breast | 1 (0.64) |
| C60-C63 | Male genital organs | 1 (0.64) |
| C64-C68 | Urinary tract | 4 (2.5) |
| C73-C75 | Endocrine glands | 1 (0.64) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alorjani, M.S.; Matalka, I.I.; Alfaqih, M.A.; Jahmani, R.A.; Alsinglawi, B.S.; Nimri, F.M.; Matalka, M.I.; Amr, S.S. Soft Tissue Sarcomas: A 16-Year Experience of a Tertiary Referral Hospital in North Jordan. Medicina 2022, 58, 198. https://doi.org/10.3390/medicina58020198
Alorjani MS, Matalka II, Alfaqih MA, Jahmani RA, Alsinglawi BS, Nimri FM, Matalka MI, Amr SS. Soft Tissue Sarcomas: A 16-Year Experience of a Tertiary Referral Hospital in North Jordan. Medicina. 2022; 58(2):198. https://doi.org/10.3390/medicina58020198
Chicago/Turabian StyleAlorjani, Mohammed S., Ismail I. Matalka, Mahmoud A. Alfaqih, Rami A. Jahmani, Belal S. Alsinglawi, Faisal M. Nimri, Mohammad I. Matalka, and Samir S. Amr. 2022. "Soft Tissue Sarcomas: A 16-Year Experience of a Tertiary Referral Hospital in North Jordan" Medicina 58, no. 2: 198. https://doi.org/10.3390/medicina58020198






