Scanning Super/Ultrapulsed CO2 Laser Efficacy in Laryngeal Malignant Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laser Equipment
2.2. Surgical Classification
2.3. Patient Selection, Clinical Work-up and Surgical Technique
3. Results
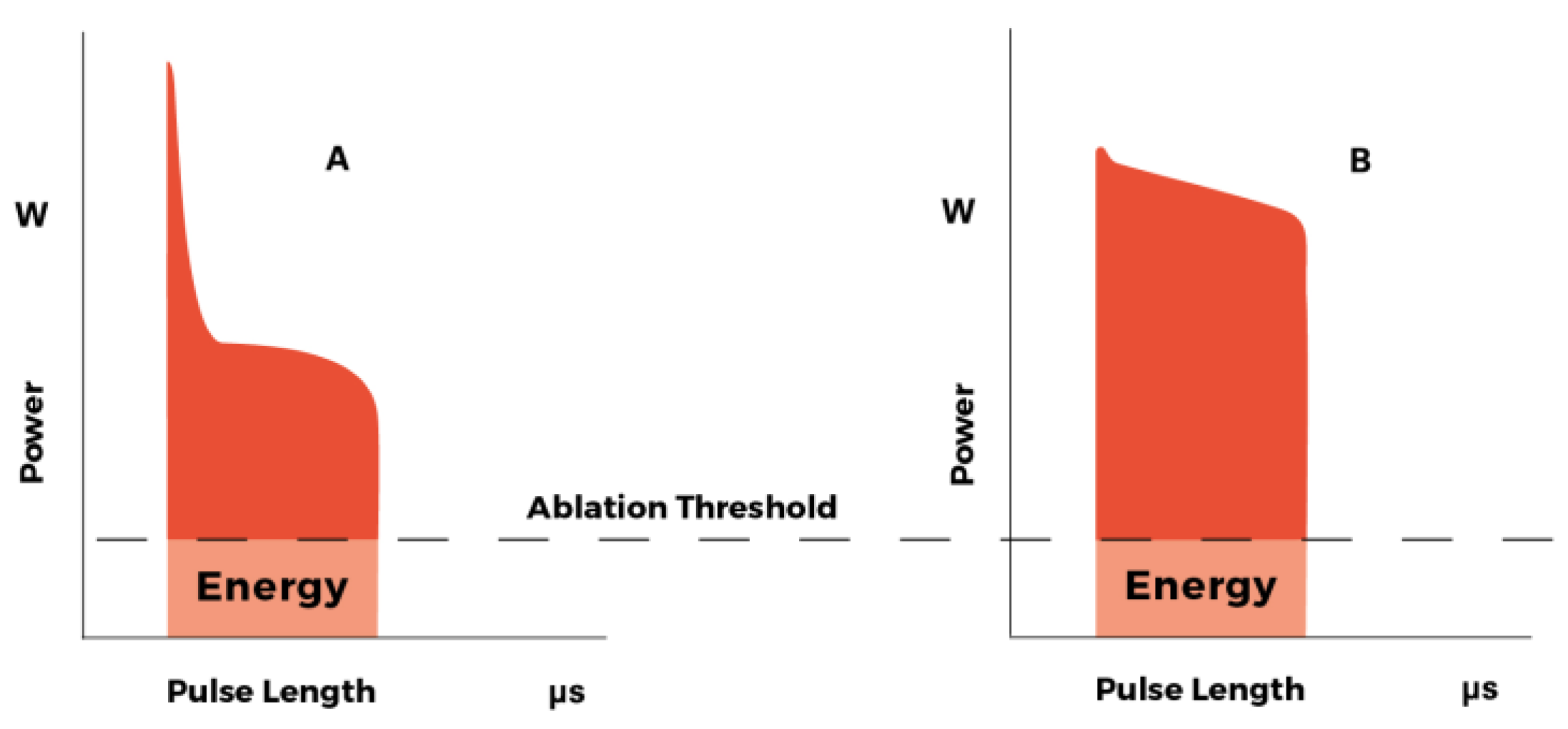
3.1. Laser Performance
3.2. Clinical Experience
3.2.1. Type I Cordectomy
3.2.2. Type II Cordectomy
3.2.3. Type III Cordectomy
3.2.4. Type IV Cordectomy
3.2.5. Type V Cordectomy
3.2.6. Type VI Cordectomy
3.2.7. Enlarged Procedures
4. Discussion
4.1. Laser Performance
4.2. Staging of the Disease
4.3. Choice of the Disease
4.4. Type of the Patients
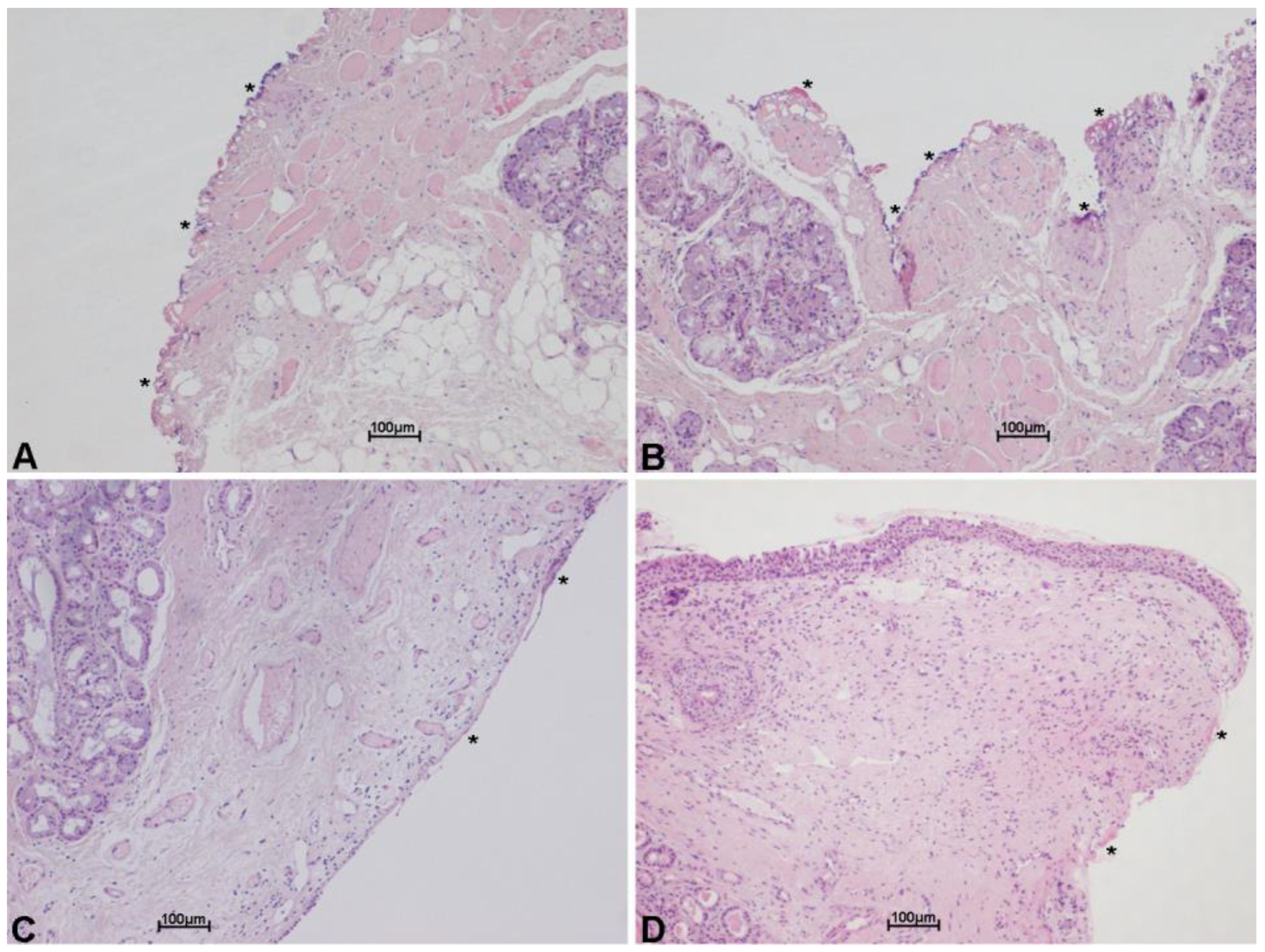
4.5. Status of the Margins
4.6. Anterior Commissure
4.7. Safety Cervicotomy
4.8. Rescue Procedures
4.9. Global Results
4.10. Follow-up
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strong, M.S.; Jako, G.J. Laser Surgery in the Larynx Early Clinical Experience with Continuous Co2 Laser. Ann. Otol. Rhinol. Laryngol. 1972, 81, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, S.P.; Bennardo, L.; Sannino, M.; Negosanti, F.; Tamburi, F.; Del Duca, E.; Giudice, A.; Cannarozzo, G. Combined CO2 and dye laser technique in the treatment of outcomes due to flap necrosis after surgery for basal cell carcinoma on the nose. Lasers Surg. Med. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cannarozzo, G.; Bennardo, L.; Negosanti, F.; Nisticò, S.P. CO2 Laser Treatment in Idiopathic Scrotal Calcinosis: A Case Series. J. Lasers Med. Sci. 2020, 11, 500–501. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.T.; Polanyi, T.G. History of the Carbon Dioxide Laser in Otolaryngologic Surgery. Otolaryngol. Clin. N. Am. 1983, 16, 739–752. [Google Scholar] [CrossRef]
- Steiner, W. Results of curative laser microsurgery of laryngeal carcinomas. Am. J. Otolaryngol. 1993, 14, 116–121. [Google Scholar] [CrossRef]
- Motta, G.; Villari, G.; Motta, G., Jr.; Ripa, G.; Salerno, G. The CO2 laser in the laryngeal microsurgery. Acta Otolaryngol. Suppl. 1986, 433, 1–30. [Google Scholar]
- Shapshay, S.M.; Hybels, R.L.; Bohigian, R.K. Laser Excision of Early Vocal Cord Carcinoma: Indications, Limitations, and Precautions. Ann. Otol. Rhinol. Laryngol. 1990, 99, 46–50. [Google Scholar] [CrossRef]
- Peretti, G.; Nicolai, P.; De Zinis, L.O.R.; Berlucchi, M.; Bazzana, T.; Bertoni, F.; Antonelli, A.R. Endoscopic CO2 Laser Excision for Tis, T1, and T2 Glottic Carcinomas: Cure Rate and Prognostic Factors. Otolaryngol. Neck Surg. 2000, 123, 124–131. [Google Scholar] [CrossRef]
- Peretti, G.; DE Zinis, L.O.R.; Nicolai, P.; Valentini, S.; Piazza, C.; Antonelli, A.R. Oncological Results of Endoscopic Resections of Tis and T1 Glottic Carcinomas by Carbon Dioxide Laser. Ann. Otol. Rhinol. Laryngol. 2001, 110, 820–826. [Google Scholar] [CrossRef]
- Remacle, M.; Lawson, G.; Nollevaux, M.-C.; Delos, M. Current State of Scanning Micromanipulator Applications with the Carbon Dioxide Laser. Ann. Otol. Rhinol. Laryngol. 2008, 117, 239–244. [Google Scholar] [CrossRef]
- Remacle, M.; Eckel, H.E.; Antonelli, A.; Brasnu, D.; Chevalier, D.; Friedrich, G.; Olofsson, J.; Rudert, H.H.; Thumfart, W.; de Vincentiiset, M.; et al. Endoscopic cordectomy. A proposal for a classification by the Working Committee, European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2000, 257, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Remacle, M.; Arens, C.; Eldin, M.B.; Campos, G.; Estomba, C.C.; Dulguerov, P.; Fiz, I.; Hantzakos, A.; Keghian, J.; Moraet, F.; et al. Laser-assisted surgery of the upper aero-digestive tract: A clarification of nomenclature. A consensus statement of the European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2017, 274, 3723–3727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remacle, M.; Van Haverbeke, C.; Eckel, H.; Bradley, P.; Chevalier, D.; Djukic, V.; de Vicentiis, M.; Friedrich, G.; Olofsson, J.; Peretti, G.; et al. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur. Arch. Otorhinolaryngol. 2007, 264, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Remacle, M.; Hantzakos, A.; Eckel, H.; Evrard, A.S.; Bradley, P.J.; Chevalier, D.; Djukic, V.; de Vincentiis, M.; Friedrich, G.; Olofssonet, J.; et al. Endoscopic supraglottic laryngectomy: A proposal for a classification by the working committee on nomenclature, European Laryngological Society. Eur. Arch. Otorhinolaryngol. 2009, 266, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Dallari, S.; Castriotta, A.; Durso, D. Chromoendoscopy in otorhinolaryngology. Experience with the SPIES® System. Otorinolaringologia 2017, 67, 81–88. [Google Scholar] [CrossRef]
- Dallari, S.; Zaraca, G.; Giorgini, S.; Borgonzoni, M. Close and positive margins in non-melanoma skin malignancies of the head and neck. What to do in patients over 75 years of age? A preliminary study. G. Ital. Dermatol. Venereol. 2020, 155, 464–498. [Google Scholar] [CrossRef]
- Dallari, S.; Zaraca, G.; Filosa, A.; Borgia, L.; Collina, G. Head and Neck Non-Melanoma Skin Malignancies and Surgical Margins in Patients Over 75 Years of Age. Which Approach to use? Further Experience and Observations. Clin. Surg. 2020, 3, 1–8. [Google Scholar]
- Hendriksma, M.; Montagne, M.W.; Langeveld, T.P.M.; Veselic, M.; Van Benthem, P.P.G.; Sjögren, E.V. Evaluation of surgical margin status in patients with early glottic cancer (Tis-T2) treated with transoral CO2 laser microsurgery, on local control. Eur. Arch. Oto-Rhino-Laryngol. 2018, 275, 2333–2340. [Google Scholar] [CrossRef] [Green Version]
- Ranstam, J.; Cook, J.A. Kaplan-Meier curve. Br. J. Surg. 2017, 104, 442. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, G.; Meccariello, G.; Deganello, A.; Maio, V.; Massi, D.; Gallo, O. Impact of low-thermal-injury devices on margin status in laryngeal cancer. An experimental ex vivo study. Oral Oncol. 2014, 50, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Mariani, C.; Carta, F.; Tatti, M.; Marrosu, V.; Gerosa, C.; Puxeddu, R. Shrinkage of specimens after CO2 laser cordectomy: An objective intraoperative evaluation. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Steiner, W.; Ambrosch, P. Endoscopic Laser Surgery of the Upper Aerodigestive Tract: With Special Emphasis on Cancer Surgery; Thieme: New York, NY, USA, 2000; Chapter 3. [Google Scholar]
- Landolfo, V.; Gervasio, C.F.; Riva, G.; Garzaro, M.; Audisio, R.; Pecorari, G.; Albera, R. Prognostic role of margin status in open and CO2 laser cordectomy for T1a-T1b glottic cancer. Braz. J. Otorhinolaryngol. 2016, 24, 30240–30243. [Google Scholar]
- Mannelli, G.; Comini, L.V.; Santoro, R.; Bettiol, A.; Vannacci, A.; Desideri, I.; Bonomo, P.; Piazza, C. T1 Glottic Cancer: Does Anterior Commissure Involvement Worsen Prognosis? Cancers 2020, 12, 1485. [Google Scholar] [CrossRef]
- Ozturk, K.; Turhal, G. Transoral Laser Surgery for Early Glottic Carcinoma: A Single Surgeon Experience of 101 Consecutive Cases. ORL J. Otorhinolaryngol. Relat. Spec. 2020, 83, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.; Piazza, C.; Cocco, D.; De Benedetto, L.; Del Bon, F.; Redaelli De Zinis, L.O.; Nicolai, P. Transoral CO2 laser treatment for Tis-T3 glottic cancer: The University of Brescia experience on 595 patients. Head Neck 2010, 32, 977–983. [Google Scholar] [CrossRef] [PubMed]



| Type I | Type II | Type III | Type IV | Type V | Type VI | Enlarged | Total | |
|---|---|---|---|---|---|---|---|---|
| Number of patients (2009–2011) | M 11 F - | M 5 F 1 | M 6 F - | M 2 F 1 | M 6 F1 | M 3 F - | M 6 F - | M 39 F 3 |
| Number of patients (2012–2016) | M 11 F - | M 4 F - | M 9 F - | M 4 F - | M 12 F - | M 6 F - | M 3 F - | M 49 F - |
| Age range | ||||||||
| <50 | 1 | 1 | - | - | - | 1 | - | 3 (3%) |
| 51–60 | 8 | 4 | 4 | 1 | 3 | 4 | - | 24 (26%) |
| 61–70 | 4 | 1 | 3 | 2 | 6 | 1 | 1 | 18 (20%) |
| 71–80 | 6 | 3 | 5 | 2 | 7 | 2 | - | 28 (29%) |
| >80 | 3 | 1 | 3 | 2 | 3 | 1 | 5 | 18 (22%) |
| Type I | Type II | Type III | Type IV | Type V | Type VI | Enlarged | Total | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 22 | 10 | 16 | 7 | 18 | 9 | 9 | 91 |
| M F | 22 - | 9 1 | 16 - | 6 1 | 17 1 | 9 - | 9 - | 88 3 |
| Histologic result after cordectomy | ||||||||
| Margins free | 12 | 8 | 10 | 5 | 8 | 5 | 5 | 53 |
| Margins close | 9 | 1 | 2 | 4 | 1 | 3 | 20 | |
| Margins involved | 1 | 1 | 4 | 2 | 5 | 3 | 1 | 17 |
| Safety Cervicotomy | 1 | 4 | 5 | 2 | 2 | 14 | ||
| Tracheotomy | 1 | 2 | 3 | 6 | ||||
| Control MLS (*) | ||||||||
| Margins free | 6 | 3 | 3 | 3 | 2 | 1 | 18 | |
| Margins close | 2 | 2 | 1 | 5 | ||||
| Margins involved | 3 | 1 | 1 | 1 | 1 | 7 | ||
| Further surgery to treat recurrence/persistence | ||||||||
| Repeated laser surg. | 1 | 2 | 2 | 5 | ||||
| + subtotal laryng. | 5 | 1 | 1 | 1 | 3 | 1 | 12 | |
| + total laryngectomy | 1 | 2 | 1 | 4 | 2 | 2 | 12 |
| Type I | Type II | Type III | Type IV | Type V | Type VI | Enlarged | Total | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 22 | 10 | 16 | 7 | 18 | 9 | 9 | 91 |
| M F | 22 - | 9 1 | 16 - | 6 1 | 17 1 | 9 - | 9 - | 88 3 |
| Oncologic result after cordectomy | ||||||||
| Locally cured with first surgery | 17 | 6 | 13 | 5 | 9 | 6 | 7 | 63 |
| Locally cured with further surgery | 5 | 4 | 3 | 2 | 8 | 3 | 2 | 27 |
| Locally non-cured (laryngeal failure) | 1 | 1 | ||||||
| Follow-up | ||||||||
| 10-year follow-up | ||||||||
| Number of patients | 11 | 5 | 7 | 3 | 7 | 3 | 6 | 42 |
| Alive NED (no evidence of disease) | 7 | 3 | 6 | 1 | 6 | 3 | 26 | |
| Alive with D | - | |||||||
| Dead of D | 1 ** | 1 ** | 2 | |||||
| Dead of other | 4 | 2 | 1 | 2 | 5 | 14 | ||
| 5-year follow-up | ||||||||
| Number of patients | 11 | 5 | 9 | 4 | 11 | 6 | 3 | 49 |
| Alive NED | 9 | 5 | 4 | 2 | 7 | 3 | 3 | 33 |
| Alive with D | - | |||||||
| Dead of D | 1 * | 1 * | 1 | 3 | ||||
| Dead of other | 1 | 4 | 2 | 4 | 2 | 13 |
| Type I | Type II | Type III | Type IV | Type V | Type VI | Enlarged | Total | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 22 | 10 | 16 | 7 | 18 | 9 | 9 | 91 |
| M F | 22 - | 9 1 | 16 - | 6 1 | 17 1 | 9 - | 9 - | 88 3 |
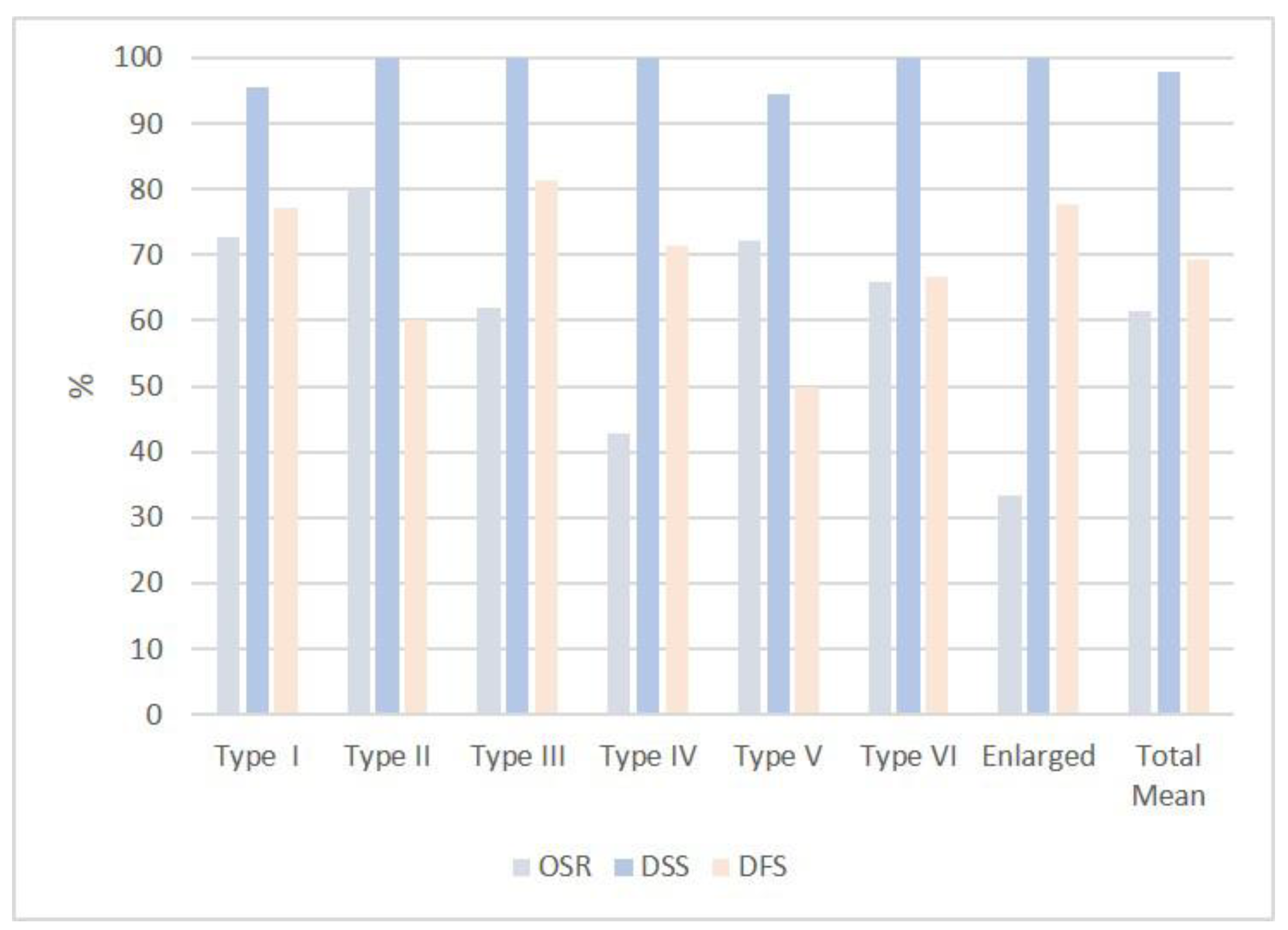
| Overall survival rate (OSR) | 16/22 (73%) | 8/10 (80%) | 10/16 (62%) | 3/7 (43%) | 13/18 (72%) | 6/9 (67%) | 3/9 (33%) | mean (61%) |
| Disease-specific surv. (DSS) | 21/22 (95%) | 10/10 (100%) | 16/16 (100%) | 7/7 (100%) | 17/18 (94%) | 9/9 (100%) | 9/9 (100%) | mean (98%) |
| Disease-free survival (DFS) | 17/22 (77%) | 6/10 (60%) | 13/16 (81%) | 5/7 (71%) | 9/18 (50%) | 6/9 (67%) | 7/9 (78%) | mean (69%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallari, S.; Giannoni, L.; Filosa, A. Scanning Super/Ultrapulsed CO2 Laser Efficacy in Laryngeal Malignant Lesions. Medicina 2022, 58, 200. https://doi.org/10.3390/medicina58020200
Dallari S, Giannoni L, Filosa A. Scanning Super/Ultrapulsed CO2 Laser Efficacy in Laryngeal Malignant Lesions. Medicina. 2022; 58(2):200. https://doi.org/10.3390/medicina58020200
Chicago/Turabian StyleDallari, Stefano, Luca Giannoni, and Alessandra Filosa. 2022. "Scanning Super/Ultrapulsed CO2 Laser Efficacy in Laryngeal Malignant Lesions" Medicina 58, no. 2: 200. https://doi.org/10.3390/medicina58020200
APA StyleDallari, S., Giannoni, L., & Filosa, A. (2022). Scanning Super/Ultrapulsed CO2 Laser Efficacy in Laryngeal Malignant Lesions. Medicina, 58(2), 200. https://doi.org/10.3390/medicina58020200






