Immune Thrombocytopenic Purpura as a Hemorrhagic Versus Thrombotic Disease: An Updated Insight into Pathophysiological Mechanisms
Abstract
1. Introduction
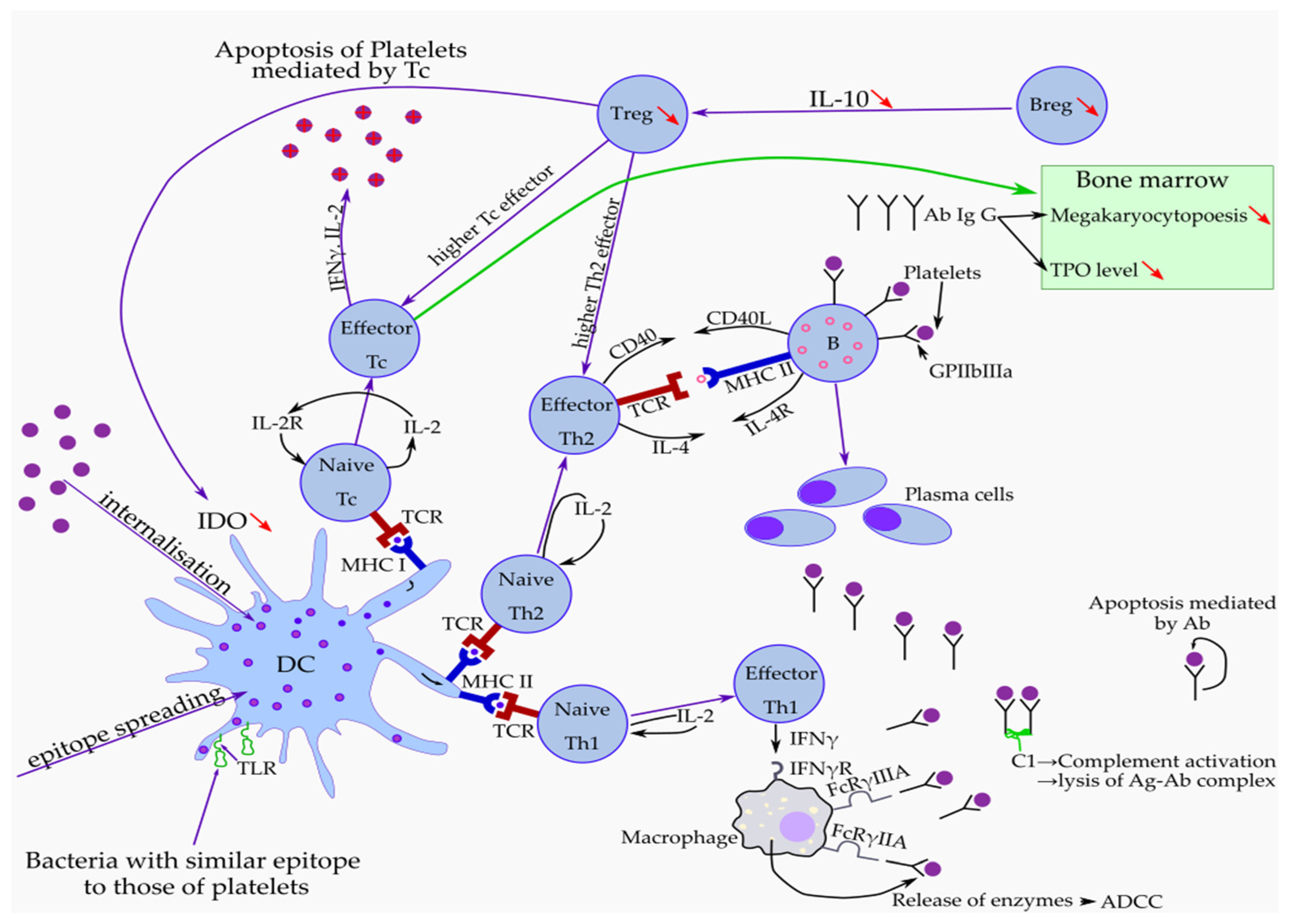
2. ITP as a Hemorrhagic Disease
Impact of Immune Response in ITP
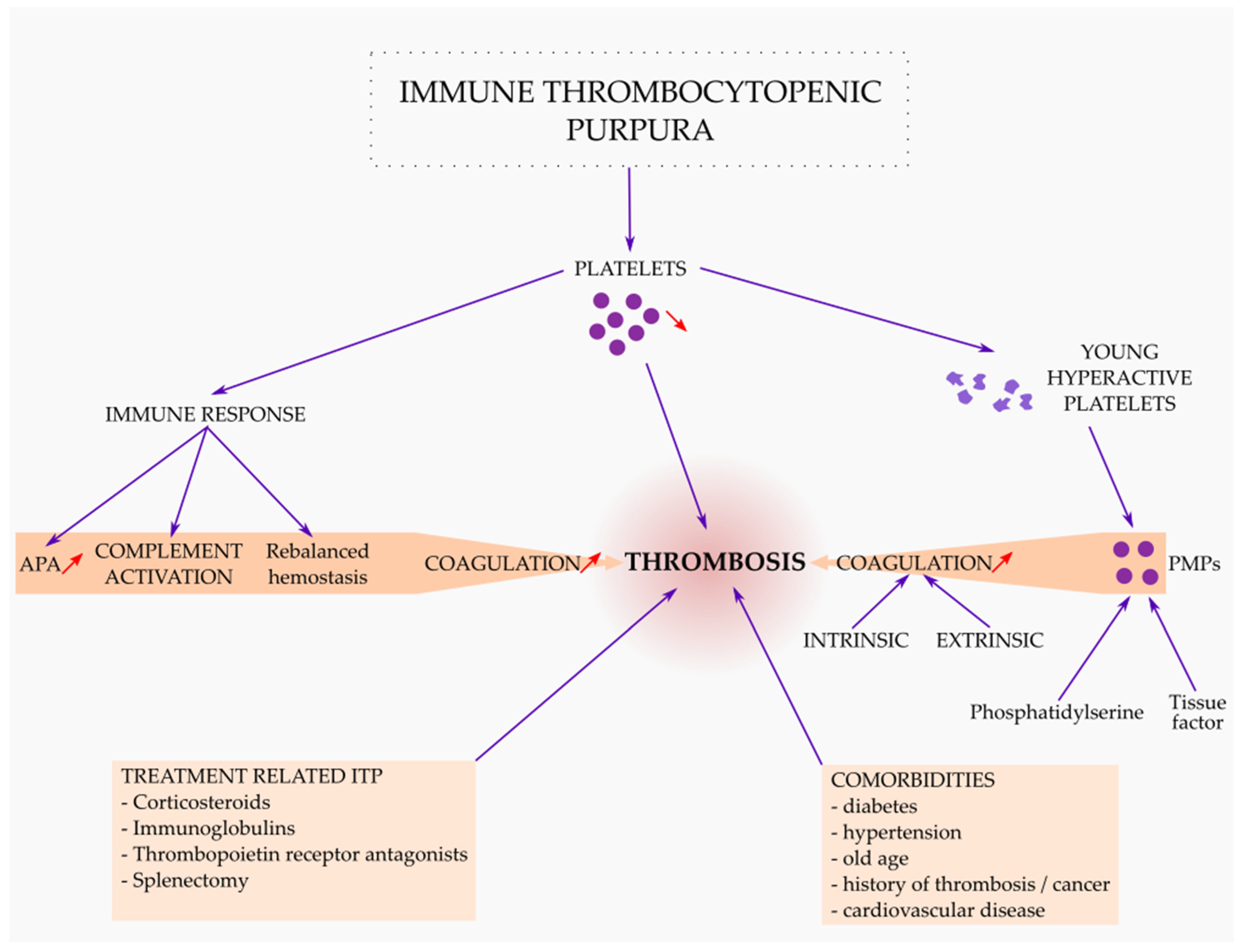
3. ITP as a Thrombotic Disease
3.1. Thrombosis Related to ITP as a Disease
3.2. Thrombosis Related to ITP Treatment
3.3. Thrombosis Related with Comorbidities in ITP Patients
3.4. The Management of Thrombotic Events in ITP
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Moulis, G.; Palmaro, A.; Montastruc, J.-L.; Godeau, B.; Lapeyre-Mestre, M.; Sailler, L. Epidemiology of Incident Immune Thrombocytopenia: A Nationwide Population-Based Study in France. Blood 2014, 124, 3308–3315. [Google Scholar] [CrossRef]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 Guidelines for Immune Thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef]
- Cines, D.B.; Liebman, H.A. The Immune Thrombocytopenia Syndrome: A Disorder of Diverse Pathogenesis and Clinical Presentation. Hematol. /Oncol. Clin. North Am. 2009, 23, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.A.; Neely, J.; Farhoudi, N. ITP in Children: Pathophysiology and Current Treatment Approaches. J. Pediatric Hematol. /Oncol. 2013, 35, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, A.; Kapur, R.; Semple, J.W. Pathogenesis and Therapeutic Mechanisms in Immune Thrombocytopenia (ITP). J. Clin. Med. 2017, 6, E16. [Google Scholar] [CrossRef]
- Lev, P.; Goette, N.; Marta, R. Pathophysiological Mechanisms Leading to Low Platelet Count in Immune Thrombocytopenia. J. Immunol. Sci. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Găman, M.-A.; Maria Găman, A. Pathophysiology, Diagnosis and Treatment of Immune Thrombocytopenia. Int. J. Med. Stud. 2017, 5, 32–36. [Google Scholar] [CrossRef]
- Bennett, C.M.; Neunert, C.; Grace, R.F.; Buchanan, G.; Imbach, P.; Vesely, S.K.; Kuhne, T. Predictors of Remission in Children with Newly Diagnosed Immune Thrombocytopenia: Data from the Intercontinental Cooperative ITP Study Group Registry II Participants. Pediatr. Blood Cancer 2018, 65, e26736. [Google Scholar] [CrossRef]
- Perera, M.; Garrido, T. Advances in the Pathophysiology of Primary Immune Thrombocytopenia. Hematology 2017, 22, 41–53. [Google Scholar] [CrossRef]
- Zhang, W.; Nardi, M.A.; Borkowsky, W.; Li, Z.; Karpatkin, S. Role of Molecular Mimicry of Hepatitis C Virus Protein with Platelet GPIIIa in Hepatitis C–Related Immunologic Thrombocytopenia. Blood 2009, 113, 4086–4093. [Google Scholar] [CrossRef]
- Zlamal, J.; Bakchoul, T. Acquired Immune Thrombocytopenia: An Update on Pathophysiology, Diagnosis and Management. Ann. Blood 2018, 3, 45. [Google Scholar] [CrossRef]
- Audia, S.; Mahévas, M.; Samson, M.; Godeau, B.; Bonnotte, B. Pathogenesis of Immune Thrombocytopenia. Autoimmun. Rev. 2017, 16, 620–632. [Google Scholar] [CrossRef]
- Consolini, R.; Legitimo, A.; Caparello, M.C. The Centenary of Immune Thrombocytopenia—Part 1: Revising Nomenclature and Pathogenesis. Front. Pediatr. 2016, 4, 102. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yujiri, T.; Tanizawa, Y. Helicobacter Pylori and Chronic ITP: The Discrepancy in the Clinical Responses to Eradication Therapy Might Be Due to Differences in the Bacterial Strains. Blood 2004, 104, 594. [Google Scholar] [CrossRef]
- Rajan, S.K.; Espina, B.M.; Liebman, H.A. Hepatitis C Virus-Related Thrombocytopenia: Clinical and Laboratory Characteristics Compared with Chronic Immune Thrombocytopenic Purpura. Br. J. Haematol. 2005, 129, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of Terminology, Definitions and Outcome Criteria in Immune Thrombocytopenic Purpura of Adults and Children: Report from an International Working Group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef]
- Singh, A.; Uzun, G.; Bakchoul, T. Primary Immune Thrombocytopenia: Novel Insights into Pathophysiology and Disease Management. JCM 2021, 10, 789. [Google Scholar] [CrossRef]
- Frelinger, A.L.; Grace, R.F.; Gerrits, A.J.; Carmichael, S.L.; Forde, E.E.; Michelson, A.D. Platelet Function in ITP, Independent of Platelet Count, Is Consistent Over Time and Is Associated with Both Current and Subsequent Bleeding Severity. Thromb. Haemost. 2018, 118, 143–151. [Google Scholar] [CrossRef]
- Blair, T.A.; Michelson, A.D.; Frelinger, A.L. Mass Cytometry Reveals Distinct Platelet Subtypes in Healthy Subjects and Novel Alterations in Surface Glycoproteins in Glanzmann Thrombasthenia. Sci. Rep. 2018, 8, 10300. [Google Scholar] [CrossRef]
- Zhai, J.; Ding, M.; Yang, T.; Zuo, B.; Weng, Z.; Zhao, Y.; He, J.; Wu, Q.; Ruan, C.; He, Y. Flow Cytometric Immunobead Assay for Quantitative Detection of Platelet Autoantibodies in Immune Thrombocytopenia Patients. J. Transl. Med. 2017, 15, 214. [Google Scholar] [CrossRef][Green Version]
- McMillan, R.; Wang, L.; Tani, P. Prospective Evaluation of the Immunobead Assay for the Diagnosis of Adult Chronic Immune Thrombocytopenic Purpura (ITP): Immunobead Assay in Chronic ITP. J. Thromb. Haemost. 2003, 1, 485–491. [Google Scholar] [CrossRef]
- Curtis, B.R. Drug-Induced Immune Thrombocytopenia: Incidence, Clinical Features, Laboratory Testing, and Pathogenic Mechanisms. Immunohematology 2019, 30, 55–65. [Google Scholar] [CrossRef]
- Marini, I.; Bakchoul, T. Pathophysiology of Autoimmune Thrombocytopenia: Current Insight with a Focus on Thrombopoiesis. Hamostaseologie 2019, 39, 227–237. [Google Scholar] [CrossRef]
- Kistangari, G.; McCrae, K.R. Immune thrombocytopenia. Hematol Oncol Clin North Am. 2013, 27, 495–520. [Google Scholar] [CrossRef]
- McKenzie, C.G.J.; Guo, L.; Freedman, J.; Semple, J.W. Cellular Immune Dysfunction in Immune Thrombocytopenia (ITP). Br. J. Haematol. 2013, 163, 10–23. [Google Scholar] [CrossRef]
- Shulman, N.R.; Marder, V.J.; Weinrach, R.S. Similarities between known antiplatelet antibodies and the factor responsible for thrombocytopenia in idiopathic purpura. Physiologic, serologic and isotopic studies. Ann. N. Y. Acad. Sci. 1965, 124, 499–542. [Google Scholar] [CrossRef] [PubMed]
- Ku, F.-C.; Tsai, C.-R.; Der Wang, J.-; Wang, C.H.; Chang, T.-K.; Hwang, W.-L. Stromal-derived factor-1 gene variations in pediatric patients with primary immune thrombocytopenia. Eur. J. Haematol. 2013, 90, 25–30. [Google Scholar] [CrossRef]
- Rank, A.; Weigert, O.; Ostermann, H. Management of chronic immune thrombocytopenic purpura: Targeting insufficient megakaryopoiesis as a novel therapeutic principle. Biologics 2010, 4, 139–145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Z.; Yang, L.; Yang, G.; Zhuang, Y.; Qian, X.; Zhou, X.; Xiao, D.; Shen, Y. Contributions of T lymphocyte abnormalities to therapeutic outcomes in newly diagnosed patients with immune thrombocytopenia. PLoS ONE 2015, 10, e0126601. [Google Scholar] [CrossRef]
- Harrington, W.J.; Minnich, V.; Hollingsworth, J.W.; Moore, C.V. Demonstration of a Thrombocytopenic Factor in the Blood of Patients with Thrombocytopenic Purpura. J. Lab. Clin. Med. 1951, 38, 1–10. [Google Scholar] [PubMed]
- Karpatkin, S.; Siskind, G.W. In Vitro Detection of Platelet Antibody in Patients with Idiopathic Thrombocytopenic Purpura and Systemic Lupus Erythematosus. Blood 1969, 33, 795–812. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Reid, D.; Jones, C.; Shulman, N. Spectrum of Ig Classes, Specificities, and Titers of Serum Antiglycoproteins in Chronic Idiopathic Thrombocytopenic Purpura. Blood 1994, 83, 1024–1032. [Google Scholar] [CrossRef]
- van Leeuwen, E.; van der Ven, J.; Engelfriet, C.; von dem Borne, A. Specificity of Autoantibodies in Autoimmune Thrombocytopenia. Blood 1982, 59, 23–26. [Google Scholar] [CrossRef]
- Hauch, T.; Rosse, W. Platelet-Bound Complement (C3) in Immune Thrombocytopenia. Blood 1977, 50, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Tsubakio, T.; Tani, P.; Curd, J.G.; Mcmillan, R. Complement Activation in Vitro by Antiplatelet Antibodies in Chronic Immune Thrombocytopenic Purpura. Br. J. Haematol. 2008, 63, 293–300. [Google Scholar] [CrossRef]
- Najaoui, A.; Bakchoul, T.; Stoy, J.; Bein, G.; Rummel, M.J.; Santoso, S.; Sachs, U.J. Autoantibody-Mediated Complement Activation on Platelets Is a Common Finding in Patients with Immune Thrombocytopenic Purpura (ITP): Complement Activation in ITP. Eur. J. Haematol. 2012, 88, 167–174. [Google Scholar] [CrossRef]
- Bakchoul, T.; Walek, K.; Krautwurst, A.; Rummel, M.; Bein, G.; Santoso, S.; Sachs, U.J. Glycosylation of Autoantibodies: Insights into the Mechanisms of Immune Thrombocytopenia. Thromb. Haemost. 2013, 110, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; van der Wal, D.E.; Zhu, G.; Xu, M.; Yougbare, I.; Ma, L.; Vadasz, B.; Carrim, N.; Grozovsky, R.; Ruan, M.; et al. Desialylation Is a Mechanism of Fc-Independent Platelet Clearance and a Therapeutic Target in Immune Thrombocytopenia. Nat. Commun. 2015, 6, 7737. [Google Scholar] [CrossRef]
- Quach, M.E.; Dragovich, M.A.; Chen, W.; Syed, A.K.; Cao, W.; Liang, X.; Deng, W.; De Meyer, S.F.; Zhu, G.; Peng, J.; et al. Fc-Independent Immune Thrombocytopenia via Mechanomolecular Signaling in Platelets. Blood 2018, 131, 787–796. [Google Scholar] [CrossRef]
- Marini, I.; Zlamal, J.; Faul, C.; Holzer, U.; Hammer, S.; Pelzl, L.; Bethge, W.; Althaus, K.; Bakchoul, T. Autoantibody-Mediated Desialylation Impairs Human Thrombopoiesis and Platelet Life Span. Haematologica 2021, 106, 196–207. [Google Scholar] [CrossRef]
- Mason, K.D.; Carpinelli, M.R.; Fletcher, J.I.; Collinge, J.E.; Hilton, A.A.; Ellis, S.; Kelly, P.N.; Ekert, P.G.; Metcalf, D.; Roberts, A.W.; et al. Programmed Anuclear Cell Death Delimits Platelet Life Span. Cell 2007, 128, 1173–1186. [Google Scholar] [CrossRef]
- van der Wal, D.E.; Gitz, E.; Du, V.X.; Lo, K.S.L.; Koekman, C.A.; Versteeg, S.; Akkerman, J.W.N. Arachidonic Acid Depletion Extends Survival of Cold-Stored Platelets by Interfering with the [Glycoprotein Ib-14-3-3] Association. Haematologica 2012, 97, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Grozovsky, R.; Begonja, A.J.; Liu, K.; Visner, G.; Hartwig, J.H.; Falet, H.; Hoffmeister, K.M. The Ashwell-Morell Receptor Regulates Hepatic Thrombopoietin Production via JAK2-STAT3 Signaling. Nat. Med. 2015, 21, 47–54. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Neves, M.A.D.; Zhu, G.; Carrim, N.; Yu, R.; Gupta, S.; Marshall, J.; Rotstein, O.; Peng, J.; et al. GPIbα Is Required for Platelet-Mediated Hepatic Thrombopoietin Generation. Blood 2018, 132, 622–634. [Google Scholar] [CrossRef] [PubMed]
- Machlus, K.R.; Italiano, J.E. The Incredible Journey: From Megakaryocyte Development to Platelet Formation. J. Cell Biol. 2013, 201, 785–796. [Google Scholar] [CrossRef]
- Broudy, V.C.; Lin, N.L.; Kaushansky, K. Thrombopoietin (c-Mpl Ligand) Acts Synergistically with Erythropoietin, Stem Cell Factor, and Interleukin-11 to Enhance Murine Megakaryocyte Colony Growth and Increases Megakaryocyte Ploidy in Vitro. Blood 1995, 85, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Simón, J.A.; Tabera, S.; Sarasquete, M.E.; Díez-Campelo, M.; Canchado, J.; Sánchez-Abarca, L.I.; Blanco, B.; Alberca, I.; Herrero-Sánchez, C.; Cañizo, C.; et al. Mesenchymal Stem Cells Are Functionally Abnormal in Patients with Immune Thrombocytopenic Purpura. Cytotherapy 2009, 11, 698–705. [Google Scholar] [CrossRef]
- McMillan, R.; Luiken, G.A.; Levy, R.; Yelenosky, R.; Longmire, R.L. Antibody against Megakaryocytes in Idiopathic Thrombocytopenic Purpura. JAMA 1978, 239, 2460–2462. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Sekine, N.; Nakatake, T. Influence of Monoclonal Antiplatelet Glycoprotein Antibodies on in Vitro Human Megakaryocyte Colony Formation and Proplatelet Formation. Blood 1999, 93, 1951–1958. [Google Scholar] [CrossRef]
- Chang, M.; Nakagawa, P.A.; Williams, S.A.; Schwartz, M.R.; Imfeld, K.L.; Buzby, J.S.; Nugent, D.J. Immune Thrombocytopenic Purpura (ITP) Plasma and Purified ITP Monoclonal Autoantibodies Inhibit Megakaryocytopoiesis in Vitro. Blood 2003, 102, 887–895. [Google Scholar] [CrossRef]
- Iraqi, M.; Perdomo, J.; Yan, F.; Choi, P.Y.-I.; Chong, B.H. Immune Thrombocytopenia: Antiplatelet Autoantibodies Inhibit Proplatelet Formation by Megakaryocytes and Impair Platelet Production in Vitro. Haematologica 2015, 100, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.F.; Chen, F.; Wang, S.; Chen, S.L.; Xu, Y.; Shen, M.Q.; Du, C.H.; Wang, C.; Kong, P.Y.; Cheng, T.M.; et al. Autoantibody against Integrin Av Β3 Contributes to Thrombocytopenia by Blocking the Migration and Adhesion of Megakaryocytes. J Thromb. Haemost. 2018, 16, 1843–1856. [Google Scholar] [CrossRef]
- Kuwana, M.; Okazaki, Y.; Kajihara, M.; Kaburaki, J.; Miyazaki, H.; Kawakami, Y.; Ikeda, Y. Autoantibody to C-Mpl (Thrombopoietin Receptor) in Systemic Lupus Erythematosus: Relationship to Thrombocytopenia with Megakaryocytic Hypoplasia. Arthritis. Rheum. 2002, 46, 2148–2159. [Google Scholar] [CrossRef]
- Li, X.; Zhong, H.; Bao, W.; Boulad, N.; Evangelista, J.; Haider, M.A.; Bussel, J.; Yazdanbakhsh, K. Defective Regulatory B-Cell Compartment in Patients with Immune Thrombocytopenia. Blood 2012, 120, 3318–3325. [Google Scholar] [CrossRef]
- Semple, J.W.; Provan, D. The Immunopathogenesis of Immune Thrombocytopenia: T Cells Still Take Center-Stage. Curr. Opin. Hematol. 2012, 19, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Stasi, R.; Cooper, N.; Del Poeta, G.; Stipa, E.; Laura Evangelista, M.; Abruzzese, E.; Amadori, S. Analysis of Regulatory T-Cell Changes in Patients with Idiopathic Thrombocytopenic Purpura Receiving B Cell-Depleting Therapy with Rituximab. Blood 2008, 112, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ge, J.; Zhao, H.; Du, W.; Xu, J.; Sui, T.; Ma, L.; Zhou, Z.; Qi, A.; Yang, R. Association of Cytotoxic T-Lymphocyte Antigen 4 Gene Polymorphisms with Idiopathic Thrombocytopenic Purpura in a Chinese Population. Platelets 2011, 22, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Cao, X.; Yu, Z.; Ruan, C. Circulating Dendritic Cells Subsets and CD4+Foxp3+ Regulatory T Cells in Adult Patients with Chronic ITP before and after Treatment with High-Dose Dexamethasome. Eur. J. Haematol. 2007, 79, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, D.; Zhu, X.; Qu, X.; Ji, C.; Hou, M. Elevated Profile of Th17, Th1 and Tc1 Cells in Patients with Immune Thrombocytopenic Purpura. Haematologica 2009, 94, 1326–1329. [Google Scholar] [CrossRef]
- Wang, X.-P.; Qiu, Y.-S.; Hao, G.-P.; Zhu, L. Levels of regulatory T cells in peripheral blood of children with idiopathic thrombocytopenic purpura. Zhongguo Dang Dai Er Ke Za Zhi 2011, 13, 282–284. [Google Scholar]
- Ji, L.; Zhan, Y.; Hua, F.; Li, F.; Zou, S.; Wang, W.; Song, D.; Min, Z.; Chen, H.; Cheng, Y. The Ratio of Treg/Th17 Cells Correlates with the Disease Activity of Primary Immune Thrombocytopenia. PLoS ONE 2012, 7, e50909. [Google Scholar] [CrossRef]
- Adly, A.A.M. Pathophysiology of immune thrombocytopenic purpura: A bird’s-eye view. Egypt J. Pediatr. Allergy. Immunol. 2014, 12, 49–61. [Google Scholar]
- Panitsas, F.P.; Theodoropoulou, M.; Kouraklis, A.; Karakantza, M.; Theodorou, G.L.; Zoumbos, N.C.; Maniatis, A.; Mouzaki, A. Adult Chronic Idiopathic Thrombocytopenic Purpura (ITP) Is the Manifestation of a Type-1 Polarized Immune Response. Blood 2004, 103, 2645–2647. [Google Scholar] [CrossRef]
- Mouzaki, A.; Theodoropoulou, M.; Gianakopoulos, I.; Vlaha, V.; Kyrtsonis, M.-C.; Maniatis, A. Expression Patterns of Th1 and Th2 Cytokine Genes in Childhood Idiopathic Thrombocytopenic Purpura (ITP) at Presentation and Their Modulation by Intravenous Immunoglobulin G (IVIg) Treatment: Their Role in Prognosis. Blood 2002, 100, 1774–1779. [Google Scholar] [CrossRef]
- Andersson, P.-O.; Olsson, A.; Wadenvik, H. Reduced Transforming Growth Factor-Beta1 Production by Mononuclear Cells from Patients with Active Chronic Idiopathic Thrombocytopenic Purpura. Br. J. Haematol. 2002, 116, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.; Haas, W. In Vivo Models of Dominant T-Cell Tolerance: Where Do We Stand Today? Trends Immunol. 2001, 22, 350–351. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, Z.; Li, H.; Chen, X.; Xu, J.; Gu, D.; Du, W.; Zheng, C.; Zhang, L.; Huang, Y.; et al. BAFF and BAFF-R of Peripheral Blood and Spleen Mononuclear Cells in Idiopathic Thrombocytopenic Purpura. Autoimmunity 2009, 42, 112–119. [Google Scholar] [CrossRef]
- Liu, H.; Ouyang, X.; Li, Y.; Zeng, H.; Wang, X.; Xie, S.; Nie, D.; Xiao, J.; Wei, J.; Wu, Y.; et al. Involvement of Levels of Toll like Receptor-4 in Monocytes, CD4+ T-Lymphocyte Subsets, and Cytokines in Patients with Immune Thrombocytopenic Purpura. Thromb. Res. 2013, 132, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.M.C.; Souza, C.; Rocha, G.A.; de Melo, F.F.; Clementino, N.C.D.; Marino, M.C.A.; Bozzi, A.; Silva, M.L.; Martins Filho, O.A.; Queiroz, D.M.M. The Levels of IL-17A and of the Cytokines Involved in Th17 Cell Commitment Are Increased in Patients with Chronic Immune Thrombocytopenia. Haematologica 2011, 96, 1560–1564. [Google Scholar] [CrossRef]
- Saito, A.; Yokohama, A.; Osaki, Y.; Ogawa, Y.; Nakahashi, H.; Toyama, K.; Mitsui, T.; Hashimoto, Y.; Koiso, H.; Uchiumi, H.; et al. Circulating Plasmacytoid Dendritic Cells in Patients with Primary and Helicobacter Pylori-Associated Immune Thrombocytopenia. Eur. J. Haematol. 2012, 88, 340–349. [Google Scholar] [CrossRef]
- Chang, D.; Ouyang, J.; Zhou, R.; Xu, J.; Chen, B.; Yang, Y.; Zhang, Q.; Shao, X.; Guan, C.; Xu, Y. Profiles of different subsets of CD(4)(+) T cells in chronic idiopathic thrombocytopenic purpura. Zhonghua Nei Ke Za Zhi 2010, 49, 213–216. [Google Scholar]
- Cao, J.; Chen, C.; Zeng, L.; Li, L.; Li, X.; Li, Z.; Xu, K. Elevated Plasma IL-22 Levels Correlated with Th1 and Th22 Cells in Patients with Immune Thrombocytopenia. Clin. Immunol. 2011, 141, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Miyara, M.; Sakaguchi, S. Natural Regulatory T Cells: Mechanisms of Suppression. Trends Mol. Med. 2007, 13, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Grohmann, U.; Hwang, K.W.; Orabona, C.; Vacca, C.; Bianchi, R.; Belladonna, M.L.; Fioretti, M.C.; Alegre, M.-L.; Puccetti, P. Modulation of Tryptophan Catabolism by Regulatory T Cells. Nat. Immunol. 2003, 4, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhu, X.; Zhao, P.; Zhao, C.; Li, X.; Zhu, Y.; Li, L.; Sun, J.; Peng, J.; Ji, C.; et al. Profile of Th17 Cytokines (IL-17, TGF-Beta, IL-6) and Th1 Cytokine (IFN-Gamma) in Patients with Immune Thrombocytopenic Purpura. Ann. Hematol. 2008, 87, 899–904. [Google Scholar] [CrossRef]
- Hemdan, N.Y.A.; Birkenmeier, G.; Wichmann, G.; Abu El-Saad, A.M.; Krieger, T.; Conrad, K.; Sack, U. Interleukin-17-Producing T Helper Cells in Autoimmunity. Autoimmun. Rev. 2010, 9, 785–792. [Google Scholar] [CrossRef]
- Li, F.; Ji, L.; Wang, W.; Hua, F.; Zhan, Y.; Zou, S.; Yuan, L.; Ke, Y.; Min, Z.; Song, D.; et al. Insufficient Secretion of IL-10 by Tregs Compromised Its Control on over-Activated CD4+ T Effector Cells in Newly Diagnosed Adult Immune Thrombocytopenia Patients. Immunol. Res. 2015, 61, 269–280. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, D.; Yang, Y.; Zhang, X.; Lv, C.; Fu, R.; Lv, M.; Liu, W.; Chen, Y.; Liu, W.; et al. Interleukin 35 May Contribute to the Loss of Immunological Self-Tolerance in Patients with Primary Immune Thrombocytopenia. Br. J. Haematol. 2015, 169, 278–285. [Google Scholar] [CrossRef]
- Nishimoto, T.; Satoh, T.; Simpson, E.K.; Ni, H.; Kuwana, M. Predominant Autoantibody Response to GPIb/IX in a Regulatory T-Cell-Deficient Mouse Model for Immune Thrombocytopenia. J. Thromb. Haemost. 2013, 11, 369–372. [Google Scholar] [CrossRef]
- Audia, S.; Rossato, M.; Santegoets, K.; Spijkers, S.; Wichers, C.; Bekker, C.; Bloem, A.; Boon, L.; Flinsenberg, T.; Compeer, E.; et al. Splenic TFH Expansion Participates in B-Cell Differentiation and Antiplatelet-Antibody Production during Immune Thrombocytopenia. Blood 2014, 124, 2858–2866. [Google Scholar] [CrossRef]
- Amodio, G.; Gregori, S. Dendritic Cells a Double-Edge Sword in Autoimmune Responses. Front. Immunol. 2012, 3, 233. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of Dendritic Cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef] [PubMed]
- Coutant, F.; Miossec, P. Altered Dendritic Cell Functions in Autoimmune Diseases: Distinct and Overlapping Profiles. Nat. Rev. Rheumatol. 2016, 12, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Catani, L.; Fagioli, M.E.; Tazzari, P.L.; Ricci, F.; Curti, A.; Rovito, M.; Preda, P.; Chirumbolo, G.; Amabile, M.; Lemoli, R.M.; et al. Dendritic Cells of Immune Thrombocytopenic Purpura (ITP) Show Increased Capacity to Present Apoptotic Platelets to T Lymphocytes. Exp. Hematol. 2006, 34, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.-X.; Huang, B.-H.; Zhang, X.-L.; Chen, L.-M.; Wang, Y.; Yu, W.-J.; Wang, X.-L.; Qin, Y.-P. Dexamethasone Inhibits Immunoreactivity of Dendritic Cells in Patients with Chronic Idiopathic Thrombocytopenic Purpura. Blood Coagul. Fibrinolysis 2010, 21, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Zhuang, L.; Li, X.-Y.; Li, J.; Luo, S.-K. The role of B cell-activating factor secreted by peripheral blood monocyte-derived dendritic cell in chronic idiopathic thrombocytopenic purpura. Zhonghua Xue Ye Xue Za Zhi 2010, 31, 599–602. [Google Scholar]
- Aslam, R.; Speck, E.R.; Kim, M.; Crow, A.R.; Bang, K.W.A.; Nestel, F.P.; Ni, H.; Lazarus, A.H.; Freedman, J.; Semple, J.W. Platelet Toll-like Receptor Expression Modulates Lipopolysaccharide-Induced Thrombocytopenia and Tumor Necrosis Factor-Alpha Production in Vivo. Blood 2006, 107, 637–641. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Han, J.; Yang, Z.; Sheng, W.; Dai, H.; Wang, Y.; Xia, T.; Hou, M. TLR7 Regulates Dendritic Cell-Dependent B-Cell Responses through BlyS in Immune Thrombocytopenic Purpura. Eur. J. Haematol. 2011, 86, 67–74. [Google Scholar] [CrossRef]
- Catani, L.; Sollazzo, D.; Trabanelli, S.; Curti, A.; Evangelisti, C.; Polverelli, N.; Palandri, F.; Baccarani, M.; Vianelli, N.; Lemoli, R.M. Decreased Expression of Indoleamine 2,3-Dioxygenase 1 in Dendritic Cells Contributes to Impaired Regulatory T Cell Development in Immune Thrombocytopenia. Ann. Hematol. 2013, 92, 67–78. [Google Scholar] [CrossRef]
- Xu, S.; Wang, C.; Zhu, X.; Dong, X.; Shi, Y.; Peng, J.; Qin, P.; Sun, J.-Z.; Guo, C.; Ni, H.; et al. Decreased Indoleamine 2,3-Dioxygenase Expression in Dendritic Cells and Role of Indoleamine 2,3-Dioxygenase-Expressing Dendritic Cells in Immune Thrombocytopenia. Ann. Hematol. 2012, 91, 1623–1631. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The Multifaceted Biology of Plasmacytoid Dendritic Cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Kolbeck, R.; Sanjuan, M.A. Plasmacytoid Dendritic Cells in Autoimmunity. Curr. Opin. Immunol. 2017, 44, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Tavassoli, M.; McMillan, R. Structure of the Spleen in Idiopathic Thrombocytopenic Purpura. Am. J. Clin. Pathol. 1975, 64, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Kuwana, M.; Okazaki, Y.; Ikeda, Y. Splenic Macrophages Maintain the Anti-Platelet Autoimmune Response via Uptake of Opsonized Platelets in Patients with Immune Thrombocytopenic Purpura. J. Thromb. Haemost. 2009, 7, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, S.; Sun, J.; Ren, J.; Shi, Y.; Sun, L.; Dong, X.; Qin, P.; Guo, C.; Hou, M.; et al. High-Dose Dexamethasone Shifts the Balance of Stimulatory and Inhibitory Fcgamma Receptors on Monocytes in Patients with Primary Immune Thrombocytopenia. Blood 2011, 117, 2061–2069. [Google Scholar] [CrossRef]
- Audia, S.; Santegoets, K.; Laarhoven, A.G.; Vidarsson, G.; Facy, O.; Ortega-Deballon, P.; Samson, M.; Janikashvili, N.; Saas, P.; Bonnotte, B.; et al. Fcγ Receptor Expression on Splenic Macrophages in Adult Immune Thrombocytopenia. Clin. Exp. Immunol. 2017, 188, 275–282. [Google Scholar] [CrossRef]
- Garcia-Suarez, J.; Prieto, A.; Reyes, E.; Manzano, L.; Merino, J.L.; Alvarez-Mon, M. Severe Chronic Autoimmune Thrombocytopenic Purpura Is Associated with an Expansion of CD56+ CD3- Natural Killer Cells Subset. Blood 1993, 82, 1538–1545. [Google Scholar] [CrossRef]
- Aledort, L.M.; Hayward, C.P.M.; Chen, M.-G.; Nichol, J.L.; Bussel, J. ITP Study Group Prospective screening of 205 patients with ITP, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am. J. Hematol. 2004, 76, 205–213. [Google Scholar] [CrossRef]
- Rasheed, M.; Soliman, A.T.; Yassin, M.A. Thrombosis in Patients with Immune Thrombocytopenia, Review of Literature. Blood 2020, 136, 9–10. [Google Scholar] [CrossRef]
- Rodeghiero, F. ITP and thrombosis: An intriguing association. Blood Adv. 2017, 1, 2280. [Google Scholar] [CrossRef]
- Langeberg, W.J.; Schoonen, W.M.; Eisen, M.; Gamelin, L.; Stryker, S. Thromboembolism in patients with immune thrombocytopenia (ITP): A meta-analysis of observational studies. Int. J. Hematol. 2016, 103, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Boulware, R.; Refaai, M.A. Why do patients with immune thrombocytopenia (ITP) experience lower bleeding events despite thrombocytopenia? Thromb. Res. 2020, 187, 154–158. [Google Scholar] [CrossRef]
- Andic, N.; Gunduz, E.; Akay, O.M.; Şahin, D.; Teke, H.Ü. Cardiac and pulmonary thrombosis during multidrug treatment in an idiopathic thrombocytopenic purpura patient. Platelets 2014, 25, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Yang, E.J.; Lim, Y.T. Dural Venous Sinus Thrombosis and Pulmonary Embolism Following Immunoglobulin Treatment in Pediatric Patient With Immune Thrombocytopenic Purpura. J. Pediatr. Hematol. Oncol. 2017, 39, e508–e511. [Google Scholar] [CrossRef]
- Han, X.; Li, C.; Zhang, S.; Hou, X.; Chen, Z.; Zhang, J.; Zhang, Y.; Sun, J.; Wang, Y. Why thromboembolism occurs in some patients with thrombocytopenia and treatment strategies. Thromb. Res. 2020, 196, 500–509. [Google Scholar] [CrossRef]
- Jy, W.; Horstman, L.L.; Arce, M.; Ahn, Y.S. Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J. Lab. Clin. Med. 1992, 119, 334–345. [Google Scholar]
- Sewify, E.M.; Sayed, D.; Abdel Aal, R.F.; Ahmad, H.M.; Abdou, M.A. Increased circulating red cell microparticles (RMP) and platelet microparticles (PMP) in immune thrombocytopenic purpura. Thromb. Res. 2013, 131, e59–e63. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Yan, M.; Cheng, Z.; Pan, X. Pulmonary Thromboembolism in Immune Thrombocytopenia: A Report of Five Cases and a Review of the Literature. Int. J. Gen. Med. 2021, 14, 4479–4483. [Google Scholar] [CrossRef]
- Jain, A.; Saluja, S.; Chaudhry, S.; Gupta, D.K. Recurrent Arterial and Venous Thrombosis in Chronic Immune Thrombocytopenia: Clinical Paradox and Therapeutic Challenges. Indian J. Hematol. Blood Transfus. 2019, 35, 590–592. [Google Scholar] [CrossRef]
- Kailashiya, J. Platelet-derived microparticles analysis: Techniques, challenges and recommendations. Anal. Biochem. 2018, 546, 78–85. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Luo, L.; Norström, E.; Braun, O.Ö.; Mörgelin, M.; Thorlacius, H. Platelet-derived microparticles regulates thrombin generation via phophatidylserine in abdominal sepsis. J. Cell Physiol. 2018, 233, 1051–1060. [Google Scholar] [CrossRef]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef]
- Owens, A.P.; Mackman, N. Microparticles in hemostasis and thrombosis. Circ. Res. 2011, 108, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, Y.; Xu, L.; Xiao, L.; Yue, Y.; Liu, S.; Huang, Q.; Li, S.; Li, Y. Diagnostic value of platelet-derived microparticles in pulmonary thromboembolism: A population-based study. Exp. Ther. Med. 2018, 16, 3099–3106. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, H.; El-Gohary, R.M.; Mahfouz, S.; Ahmed, H.M.A.; El Demerdash, D.M.; Ragab, G. Multifocal avascular necrosis in a patient with refractory immune thrombocytopenia and antiphospholipid antibodies; case report and review of literature. Platelets 2019, 30, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Habe, K.; Wada, H.; Matsumoto, T.; Ohishi, K.; Ikejiri, M.; Matsubara, K.; Morioka, T.; Kamimoto, Y.; Ikeda, T.; Katayama, N.; et al. Presence of Antiphospholipid Antibodies as a Risk Factor for Thrombotic Events in Patients with Connective Tissue Diseases and Idiopathic Thrombocytopenic Purpura. Intern. Med. 2016, 55, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Tomasello, R.; Giordano, G.; Romano, F.; Vaccarino, F.; Siragusa, S.; Lucchesi, A.; Napolitano, M. Immune Thrombocytopenia in Antiphospholipid Syndrome: Is It Primary or Secondary? Biomedicines 2021, 9, 1170. [Google Scholar] [CrossRef] [PubMed]
- Hisada, R.; Kato, M.; Sugawara, E.; Fujieda, Y.; Oku, K.; Bohgaki, T.; Amengual, O.; Yasuda, S.; Atsumi, T. Thrombotic risk stratification by platelet count in patients with antiphospholipid antibodies: A longitudinal study. J. Thromb. Haemost. 2017, 15, 1782–1787. [Google Scholar] [CrossRef]
- Garabet, L.; Henriksson, C.E.; Lozano, M.L.; Ghanima, W.; Bussel, J.; Brodin, E.; Fernández-Pérez, M.P.; Martínez, C.; González-Conejero, R.; Mowinckel, M.-C.; et al. Markers of endothelial cell activation and neutrophil extracellular traps are elevated in immune thrombocytopenia but are not enhanced by thrombopoietin receptor agonists. Thromb. Res. 2020, 185, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Efat, A.; Shoeib, S.; Nazir, A.; Abdelmohsen, E.; Dawod, A.; Bedair, H.M.; Elgheriany, W. Endothelial Activation and Immune Thrombocytopenia: An Engagement Waiting for Consolidation. Clin. Appl. Thromb. Hemost. 2021, 27, 10760296211054514. [Google Scholar] [CrossRef]
- Kim, W.H.; Park, J.B.; Jung, C.W.; Kim, G.S. Rebalanced hemostasis in patients with idiopathic thrombocytopenic purpura. Platelets 2015, 26, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hamad, O.A.; Ekdahl, K.N.; Nilsson, P.H.; Andersson, J.; Magotti, P.; Lambris, J.D.; Nilsson, B. Complement activation triggered by chondroitin sulfate released by thrombin receptor-activated platelets. J. Thromb. Haemost. 2008, 6, 1413–1421. [Google Scholar] [CrossRef]
- Luo, S.; Hu, D.; Wang, M.; Zipfel, P.F.; Hu, Y. Complement in Hemolysis- and Thrombosis- Related Diseases. Front. Immunol. 2020, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Gulla, K.C.; Gupta, K.; Krarup, A.; Gal, P.; Schwaeble, W.J.; Sim, R.B.; O’Connor, C.D.; Hajela, K. Activation of mannan-binding lectin-associated serine proteases leads to generation of a fibrin clot. Immunology 2010, 129, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Al-Samkari, H. Management of Adult Patients with Immune Thrombocytopenia (ITP): A Review on Current Guidance and Experience from Clinical Practice. J. Blood. Med. 2021, 12, 653–664. [Google Scholar] [CrossRef]
- Moulis, G.; Germain, J.; Comont, T.; Brun, N.; Dingremont, C.; Castel, B.; Arista, S.; Sailler, L.; Lapeyre-Mestre, M.; Beyne-Rauzy, O.; et al. Newly diagnosed immune thrombocytopenia adults: Clinical epidemiology, exposure to treatments, and evolution. Results of the CARMEN multicenter prospective cohort. Am. J. Hematol. 2017, 92, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Al-Samkari, H.; Kuter, D.J. Immune Thrombocytopenia in Adults: Modern Approaches to Diagnosis and Treatment. Semin. Thromb. Hemost. 2020, 46, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.; Ghanima, W. Immune Thrombocytopenia. N. Engl. J. Med. 2019, 381, 945–955. [Google Scholar] [CrossRef]
- Girolami, A.; de Marinis, G.B.; Bonamigo, E.; Treleani, M.; Vettore, S. Arterial and venous thromboses in patients with idiopathic (immunological) thrombocytopenia: A possible contributing role of cortisone-induced hypercoagulable state. Clin. Appl. Thromb. Hemost. 2013, 19, 613–618. [Google Scholar] [CrossRef]
- Rungjirajittranon, T.; Owattanapanich, W. A serious thrombotic event in a patient with immune thrombocytopenia requiring intravenous immunoglobulin: A case report. J. Med. Case. Rep. 2019, 13, 25. [Google Scholar] [CrossRef]
- Marie, I.; Maurey, G.; Hervé, F.; Hellot, M.-F.; Levesque, H. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br. J. Dermatol. 2006, 155, 714–721. [Google Scholar] [CrossRef]
- Rhee, H.Y.; Choi, H.-Y.; Kim, S.-B.; Shin, W.-C. Recurrent ischemic stroke in a patient with idiopathic thrombocytopenic purpura. J. Thromb. Thrombolysis. 2010, 30, 229–232. [Google Scholar] [CrossRef]
- Ghanima, W.; Cooper, N.; Rodeghiero, F.; Godeau, B.; Bussel, J.B. Thrombopoietin receptor agonists: Ten years later. Haematologica 2019, 104, 1112–1123. [Google Scholar] [CrossRef]
- Di Buduo, C.A.; Currao, M.; Pecci, A.; Kaplan, D.L.; Balduini, C.L.; Balduini, A. Revealing eltrombopag’s promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica 2016, 101, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Tsai, Y.; Nowak, I.; Liesveld, J.; Chen, Y. Eltrombopag, a thrombopoietin receptor agonist, enhances human umbilical cord blood hematopoietic stem/primitive progenitor cell expansion and promotes multi-lineage hematopoiesis. Stem. Cell. Res. 2012, 9, 77–86. [Google Scholar] [CrossRef]
- Rodeghiero, F. Is ITP a thrombophilic disorder? Am. J. Hematol. 2016, 91, 39–45. [Google Scholar] [CrossRef]
- Kuter, D.J.; Tarantino, M.D.; Lawrence, T. Clinical overview and practical considerations for optimizing romiplostim therapy in patients with immune thrombocytopenia. Blood Rev. 2021, 49, 100811. [Google Scholar] [CrossRef] [PubMed]
- Justo Sanz, R.; Monzón Manzano, E.; Fernández Bello, I.; Teresa Álvarez Román, M.; Martín Salces, M.; Rivas Pollmar, M.I.; Jiménez Yuste, V.; Butta, N.V. Platelet Apoptosis and PAI-1 are Involved in the Pro-Coagulant State of Immune Thrombocytopaenia Patients Treated with Thrombopoietin Receptor Agonists. Thromb. Haemost. 2019, 119, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Garabet, L.; Ghanima, W.; Monceyron Jonassen, C.; Skov, V.; Holst, R.; Mowinckel, M.-C.; Hasselbalch, C.H.; Kruse, A.T.; Thomassen, M.; Liebman, H.; et al. Effect of thrombopoietin receptor agonists on markers of coagulation and P-selectin in patients with immune thrombocytopenia. Platelets 2019, 30, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Provan, D.; Newland, A.C. Current Management of Primary Immune Thrombocytopenia. Adv. Ther. 2015, 32, 875–887. [Google Scholar] [CrossRef]
- Boyle, S.; White, R.H.; Brunson, A.; Wun, T. Splenectomy and the incidence of venous thromboembolism and sepsis in patients with immune thrombocytopenia. Blood 2013, 121, 4782–4790. [Google Scholar] [CrossRef] [PubMed]
- Doobaree, I.U.; Nandigam, R.; Bennett, D.; Newland, A.; Provan, D. Thromboembolism in adults with primary immune thrombocytopenia: A systematic literature review and meta-analysis. Eur. J. Haematol. 2016, 97, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Crary, S.E.; Buchanan, G.R. Vascular complications after splenectomy for hematologic disorders. Blood 2009, 114, 2861–2868. [Google Scholar] [CrossRef]
- Pommerening, M.J.; Rahbar, E.; Minei, K.; Holcomb, J.B.; Wade, C.E.; Schreiber, M.A.; Cohen, M.J.; Underwood, S.J.; Nelson, M.; Cotton, B.A. Splenectomy is associated with hypercoagulable thrombelastography values and increased risk of thromboembolism. Surgery 2015, 158, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Buzelé, R.; Barbier, L.; Sauvanet, A.; Fantin, B. Medical complications following splenectomy. J. Visc. Surg. 2016, 153, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kawanaka, H.; Akahoshi, T.; Kinjo, N.; Iguchi, T.; Ninomiya, M.; Yamashita, Y.-I.; Ikegami, T.; Yoshizumi, T.; Shirabe, K.; Maehara, Y. Effect of laparoscopic splenectomy on portal haemodynamics in patients with liver cirrhosis and portal hypertension. Br. J. Surg. 2014, 101, 1585–1593. [Google Scholar] [CrossRef]
- Lafaurie, M.; Maquet, J.; Baricault, B.; Ekstrand, C.; Christiansen, C.F.; Linder, M.; Bahmanyar, S.; Nørgaard, M.; Sailler, L.; Lapeyre-Mestre, M.; et al. Risk factors of hospitalisation for thrombosis in adults with primary immune thrombocytopenia, including disease-specific treatments: A French nationwide cohort study. Br. J. Haematol. 2021, 195, 456–465. [Google Scholar] [CrossRef]
- Ito, S.; Fujiwara, S.-I.; Ikeda, T.; Toda, Y.; Mashima, K.; Umino, K.; Minakata, D.; Nakano, H.; Yamasaki, R.; Kawasaki, Y.; et al. Evaluation of thrombotic events in patients with immune thrombocytopenia. Ann. Hematol. 2020, 99, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Neunert, C.; Lim, W.; Crowther, M.; Cohen, A.; Solberg, L.; Crowther, M.A. American Society of Hematology The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood 2011, 117, 4190–4207. [Google Scholar] [CrossRef]
- Samuelson, B.T.; Gernsheimer, T.; Estey, E.; Garcia, D.A. Variability in management of hematologic malignancy patients with venous thromboembolism and chemotherapy-induced thrombocytopenia. Thromb. Res. 2016, 141, 104–105. [Google Scholar] [CrossRef][Green Version]
- Pishko, A.M.; Misgav, M.; Cuker, A.; Cines, D.B.; George, J.N.; Vesely, S.K.; Terrell, D.R. Management of antithrombotic therapy in adults with immune thrombocytopenia (ITP): A survey of ITP specialists and general hematologist-oncologists. J. Thromb. Thrombolysis 2018, 46, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Yavaşoğlu, I.; Yassin, M.A.D.; Tarkun, P.; Yoon, S.-S.; Samra, A.M.; Angchaisuksiri, P.; Pilipovici, V. Assessment of eltrombopag in patients with chronic immune thrombocytopenia under routine clinical practice in the Middle East, Turkey, Asia, and Australia. HemaSphere 2019, 3 (Suppl. S1), 305. [Google Scholar] [CrossRef]
- Al-Samkari, H.; Kuter, D.J. Optimal use of thrombopoietin receptor agonists in immune thrombocytopenia. Ther. Adv. Hematol. 2019, 10, 2040620719841735. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tărniceriu, C.C.; Hurjui, L.L.; Florea, I.D.; Hurjui, I.; Gradinaru, I.; Tanase, D.M.; Delianu, C.; Haisan, A.; Lozneanu, L. Immune Thrombocytopenic Purpura as a Hemorrhagic Versus Thrombotic Disease: An Updated Insight into Pathophysiological Mechanisms. Medicina 2022, 58, 211. https://doi.org/10.3390/medicina58020211
Tărniceriu CC, Hurjui LL, Florea ID, Hurjui I, Gradinaru I, Tanase DM, Delianu C, Haisan A, Lozneanu L. Immune Thrombocytopenic Purpura as a Hemorrhagic Versus Thrombotic Disease: An Updated Insight into Pathophysiological Mechanisms. Medicina. 2022; 58(2):211. https://doi.org/10.3390/medicina58020211
Chicago/Turabian StyleTărniceriu, Claudia Cristina, Loredana Liliana Hurjui, Irina Daniela Florea, Ion Hurjui, Irina Gradinaru, Daniela Maria Tanase, Carmen Delianu, Anca Haisan, and Ludmila Lozneanu. 2022. "Immune Thrombocytopenic Purpura as a Hemorrhagic Versus Thrombotic Disease: An Updated Insight into Pathophysiological Mechanisms" Medicina 58, no. 2: 211. https://doi.org/10.3390/medicina58020211
APA StyleTărniceriu, C. C., Hurjui, L. L., Florea, I. D., Hurjui, I., Gradinaru, I., Tanase, D. M., Delianu, C., Haisan, A., & Lozneanu, L. (2022). Immune Thrombocytopenic Purpura as a Hemorrhagic Versus Thrombotic Disease: An Updated Insight into Pathophysiological Mechanisms. Medicina, 58(2), 211. https://doi.org/10.3390/medicina58020211








