Serum Uric Acid Levels in Parkinson’s Disease: A Cross-Sectional Electronic Medical Record Database Study from a Tertiary Referral Centre in Romania
Abstract
:1. Introduction
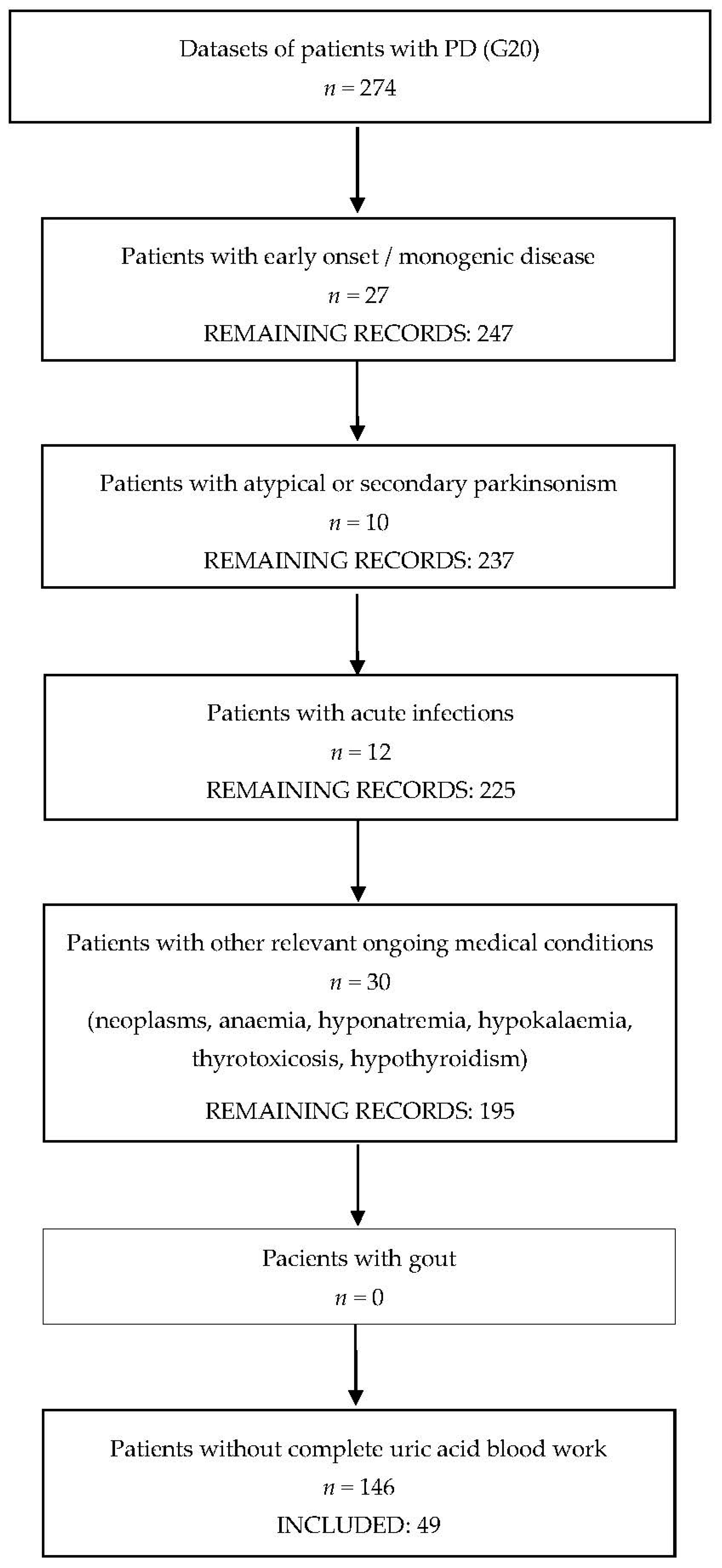
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collaborators, G.B.D.P.s.D. Global, regional, and national burden of Parkinson’s disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 939–953. [Google Scholar] [CrossRef]
- Moustafa, A.A.; Chakravarthy, S.; Phillips, J.R.; Gupta, A.; Keri, S.; Polner, B.; Frank, M.J.; Jahanshahi, M. Motor symptoms in Parkinson’s disease: A unified framework. NeuroSci. Biobehav. Rev. 2016, 68, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Solla, P.; Masala, C.; Pinna, I.; Ercoli, T.; Loy, F.; Orofino, G.; Fadda, L.; Defazio, G. Frequency and Determinants of Olfactory Hallucinations in Parkinson’s Disease Patients. Brain Sci. 2021, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor featuRes. of Parkinson disease. Nat Rev. NeuroSci. 2017, 18, 509. [Google Scholar] [CrossRef]
- Blauwendraat, C.; Nalls, M.A.; Singleton, A.B. The genetic architecture of Parkinson’s disease. Lancet Neurol. 2020, 19, 170–178. [Google Scholar] [CrossRef]
- Braak, H.; de Vos, R.A.; Bohl, J.; Del Tredici, K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. NeuroSci. Lett. 2006, 396, 67–72. [Google Scholar] [CrossRef]
- Hawkes, C.H.; Del Tredici, K.; Braak, H. Parkinson’s disease: The dual hit theory revisited. Ann. NY Acad. Sci. 2009, 1170, 615–622. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- El Ridi, R.; Tallima, H. Physiological functions and pathogenic potential of uric acid: A review. J. Adv. Res. 2017, 8, 487–493. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Khan, M.A.; Shahreyar, Z.A.; Ahmed, H. Lauric acid: Its role in behavioral modulation, neuro-inflammatory and oxidative stress markers in haloperidol induced Parkinson’s disease. Pak. J. Pharm. Sci. 2020, 33, 755–763. [Google Scholar]
- Ellmore, T.M.; Suescun, J.; Castriotta, R.J.; Schiess, M.C. A Study of the Relationship Between Uric Acid and Substantia Nigra Brain Connectivity in Patients With REM Sleep Behavior Disorder and Parkinson’s Disease. Front. Neurol. 2020, 11, 815. [Google Scholar] [CrossRef]
- Church, W.H.; Ward, V.L. Uric acid is reduced in the substantia nigra in Parkinson’s disease: Effect on dopamine oxidation. Brain Res. Bull 1994, 33, 419–425. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rub, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Musgrove, R.E.; Helwig, M.; Bae, E.J.; Aboutalebi, H.; Lee, S.J.; Ulusoy, A.; Di Monte, D.A. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular alpha-synuclein transfer. J. Clin. Investig. 2019, 129, 3738–3753. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cheng, Y.; Wang, X.; Upreti, B.; Cui, R.; Liu, S.; Shan, B.; Yu, H.; Luo, C.; Xu, J. Gout Is Not Just Arthritis: Abnormal Cortical Thickness and Structural Covariance Networks in Gout. Front. Neurol. 2021, 12, 662497. [Google Scholar] [CrossRef]
- Buzas, R.; Tautu, O.F.; Dorobantu, M.; Ivan, V.; Lighezan, D. Serum uric acid and arterial hypertension-Data from Sephar III survey. PLoS ONE 2018, 13, e0199865. [Google Scholar] [CrossRef]
- Sanchez-Lozada, L.G.; Rodriguez-Iturbe, B.; Kelley, E.E.; Nakagawa, T.; Madero, M.; Feig, D.I.; Borghi, C.; Piani, F.; Cara-Fuentes, G.; Bjornstad, P.; et al. Uric Acid and Hypertension: An Update With Recommendations. Am. J. Hypertens. 2020, 33, 583–594. [Google Scholar] [CrossRef]
- Kanbay, M.; Jensen, T.; Solak, Y.; Le, M.; Roncal-Jimenez, C.; Rivard, C.; Lanaspa, M.A.; Nakagawa, T.; Johnson, R.J. Uric acid in metabolic syndrome: From an innocent bystander to a central player. Eur. J. Intern. Med. 2016, 29, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Bardin, T.; Richette, P. Uric acid and cognitive decline: A double-edge sword? Curr. Opin. Rheumatol 2018, 30, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.W.; Grandinetti, A.; Waslien, C.I.; Ross, G.W.; White, L.R.; Morens, D.M. Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am. J. Epidemiol. 1996, 144, 480–484. [Google Scholar] [CrossRef]
- de Lau, L.M.; Koudstaal, P.J.; Hofman, A.; Breteler, M.M. Serum uric acid levels and the risk of Parkinson disease. Ann. Neurol. 2005, 58, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Luo, F.F.; Wei, L.; Lei, M.; Li, G.F.; Liu, Z.L.; Le, W.D.; Xu, P.Y. Association of serum uric acid levels with the progression of Parkinson’s disease in Chinese patients. Chin. Med. J. 2012, 125, 583–587. [Google Scholar] [PubMed]
- Weisskopf, M.G.; O’Reilly, E.; Chen, H.; Schwarzschild, M.A.; Ascherio, A. Plasma urate and risk of Parkinson’s disease. Am. J. Epidemiol. 2007, 166, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Annanmaki, T.; Muuronen, A.; Murros, K. Low plasma uric acid level in Parkinson’s disease. Mov. Disord. 2007, 22, 1133–1137. [Google Scholar] [CrossRef]
- Jesus, S.; Perez, I.; Caceres-Redondo, M.T.; Carrillo, F.; Carballo, M.; Gomez-Garre, P.; Mir, P. Low serum uric acid concentration in Parkinson’s disease in southern Spain. Eur. J. Neurol. 2013, 20, 208–210. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Wang, J.; Fang, F.; Cheng, G.; Jiang, Y.; Xiao, H.; Wan, Q. Impact of serum uric acid, albumin and their interaction on Parkinson’s disease. Neurol. Sci. 2017, 38, 331–336. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, J.; Ma, J.; Wang, Z.; Sun, W.; Li, M.; Huang, S.; Hu, S. Low serum uric acid levels are associated with the nonmotor symptoms and brain gray matter volume in Parkinson’s disease. Neurol. Sci. 2021. [Google Scholar] [CrossRef]
- Chen, H.; Mosley, T.H.; Alonso, A.; Huang, X. Plasma urate and Parkinson’s disease in the Atherosclerosis Risk in Communities (ARIC) study. Am. J. Epidemiol. 2009, 169, 1064–1069. [Google Scholar] [CrossRef]
- Winquist, A.; Steenland, K.; Shankar, A. Higher serum uric acid associated with decreased Parkinson’s disease prevalence in a large community-based survey. Mov. Disord. 2010, 25, 932–936. [Google Scholar] [CrossRef]
- Cortese, M.; Riise, T.; Engeland, A.; Ascherio, A.; Bjornevik, K. Urate and the risk of Parkinson’s disease in men and women. Parkinsonism Relat. Disord. 2018, 52, 76–82. [Google Scholar] [CrossRef]
- Kobylecki, C.J.; Nordestgaard, B.G.; Afzal, S. Plasma urate and risk of Parkinson’s disease: A mendelian randomization study. Ann. Neurol. 2018, 84, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Facheris, M.F.; Hicks, A.A.; Minelli, C.; Hagenah, J.M.; Kostic, V.; Campbell, S.; Hayward, C.; Volpato, C.B.; Pattaro, C.; Vitart, V.; et al. Variation in the uric acid transporter gene SLC2A9 and its association with AAO of Parkinson’s disease. J. Mol. NeuroSci. 2011, 43, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Aramburu, I.; Sanchez-Juan, P.; Jesus, S.; Gorostidi, A.; Fernandez-Juan, E.; Carrillo, F.; Sierra, M.; Gomez-Garre, P.; Caceres-Redondo, M.T.; Berciano, J.; et al. Genetic variability related to serum uric acid concentration and risk of Parkinson’s disease. Mov. Disord. 2013, 28, 1737–1740. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Ross, G.W.; Petrovitch, H.; White, L.R.; Masaki, K.H.; Nelson, J.S.; Tanner, C.M.; Curb, J.D.; Blanchette, P.L.; Abbott, R.D. Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology 2005, 64, 1047–1051. [Google Scholar] [CrossRef]
- Abbott, R.D.; Ross, G.W.; Petrovitch, H.; Masaki, K.H.; Launer, L.J.; Nelson, J.S.; White, L.R.; Tanner, C.M. Midlife milk consumption and substantia nigra neuron density at death. Neurology 2016, 86, 512–519. [Google Scholar] [CrossRef]
- Crotty, G.F.; Ascherio, A.; Schwarzschild, M.A. Targeting urate to reduce oxidative stress in Parkinson disease. Exp. Neurol. 2017, 298, 210–224. [Google Scholar] [CrossRef]
- Singh, J.A.; Cleveland, J.D. Gout and the risk of Parkinson’s disease in older adults: A study of U.S. Medicare data. BMC Neurol. 2019, 19, 4. [Google Scholar] [CrossRef]
- Hu, L.Y.; Yang, A.C.; Lee, S.C.; You, Z.H.; Tsai, S.J.; Hu, C.K.; Shen, C.C. Risk of Parkinson’s disease following gout: A population-based retrospective cohort study in Taiwan. BMC Neurol. 2020, 20, 338. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, I.A.; Kim, A.; Kang, G. Clinical Association between Gout and Parkinson’s Disease: A Nationwide Population-Based Cohort Study in Korea. Medicina 2021, 57, 1292. [Google Scholar] [CrossRef]
- Ungprasert, P.; Srivali, N.; Thongprayoon, C. Gout is not associated with a lower risk of Parkinson’s disease: A systematic review and meta-analysis. Parkinsonism Relat. Disord. 2015, 21, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- De Vera, M.; Rahman, M.M.; Rankin, J.; Kopec, J.; Gao, X.; Choi, H. Gout and the risk of Parkinson’s disease: A cohort study. Arthritis Rheum. 2008, 59, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Rodriguez, L.A.; Logroscino, G.; Hernan, M.A. Gout and risk of Parkinson disease: A prospective study. Neurology 2007, 69, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Kim, J.S.; Yoo, S.W.; Hwang, E.J.; Lyoo, C.H.; Lee, K.S. Gender difference in the effect of uric acid on striatal dopamine in early Parkinson’s disease. Eur. J. Neurol. 2020, 27, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Huertas, I.; Jesus, S.; Lojo, J.A.; Garcia-Gomez, F.J.; Caceres-Redondo, M.T.; Oropesa-Ruiz, J.M.; Carrillo, F.; Vargas-Gonzalez, L.; Martin Rodriguez, J.F.; Gomez-Garre, P.; et al. Lower levels of uric acid and striatal dopamine in non-tremor dominant Parkinson’s disease subtype. PLoS ONE 2017, 12, e0174644. [Google Scholar] [CrossRef] [PubMed]
- Ou, R.; Cao, B.; Wei, Q.; Hou, Y.; Xu, Y.; Song, W.; Zhao, B.; Shang, H. Serum uric acid levels and freezing of gait in Parkinson’s disease. Neurol. Sci. 2017, 38, 955–960. [Google Scholar] [CrossRef]
- Pellecchia, M.T.; Savastano, R.; Moccia, M.; Picillo, M.; Siano, P.; Erro, R.; Vallelunga, A.; Amboni, M.; Vitale, C.; Santangelo, G.; et al. Lower serum uric acid is associated with mild cognitive impairment in early Parkinson’s disease: A 4-year follow-up study. J. Neural Transm. 2016, 123, 1399–1402. [Google Scholar] [CrossRef]
- Moccia, M.; Picillo, M.; Erro, R.; Vitale, C.; Longo, K.; Amboni, M.; Santangelo, G.; Spina, E.; De Rosa, A.; De Michele, G.; et al. Is serum uric acid related to non-motor symptoms in de-novo Parkinson’s disease patients? Parkinsonism Relat. Disord. 2014, 20, 772–775. [Google Scholar] [CrossRef]
- Huang, X.; Ng, S.Y.; Chia, N.S.; Acharyya, S.; Setiawan, F.; Lu, Z.H.; Ng, E.; Tay, K.Y.; Au, W.L.; Tan, E.K.; et al. Serum uric acid level and its association with motor subtypes and non-motor symptoms in early Parkinson’s disease: PALS study. Parkinsonism Relat. Disord. 2018, 55, 50–54. [Google Scholar] [CrossRef]
- Shen, L.; Ji, H.F. Low uric acid levels in patients with Parkinson’s disease: Evidence from meta-analysis. BMJ Open 2013, 3, e003620. [Google Scholar] [CrossRef]
- Wen, M.; Zhou, B.; Chen, Y.H.; Ma, Z.L.; Gou, Y.; Zhang, C.L.; Yu, W.F.; Jiao, L. Serum uric acid levels in patients with Parkinson’s disease: A meta-analysis. PLoS ONE 2017, 12, e0173731. [Google Scholar] [CrossRef]
- Shen, C.; Guo, Y.; Luo, W.; Lin, C.; Ding, M. Serum urate and the risk of Parkinson’s disease: Results from a meta-analysis. Can J. Neurol. Sci. 2013, 40, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Grazynska, A.; Adamczewska, K.; Antoniuk, S.; Bien, M.; Tos, M.; Kufel, J.; Urbas, W.; Siuda, J. The Influence of Serum Uric Acid Level on Non-Motor Symptoms Occurrence and Severity in Patients with Idiopathic Parkinson’s Disease and Atypical Parkinsonisms-A Systematic Review. Medicina 2021, 57, 972. [Google Scholar] [CrossRef] [PubMed]
- Andreadou, E.; Nikolaou, C.; Gournaras, F.; Rentzos, M.; Boufidou, F.; Tsoutsou, A.; Zournas, C.; Zissimopoulos, V.; Vassilopoulos, D. Serum uric acid levels in patients with Parkinson’s disease: Their relationship to treatment and disease duration. Clin. Neurol. Neurosurg. 2009, 111, 724–728. [Google Scholar] [CrossRef]
- Kim, H.N.; Shin, J.Y.; Kim, D.Y.; Lee, J.E.; Lee, P.H. Priming mesenchymal stem cells with uric acid enhances neuroprotective properties in parkinsonian models. J. Tissue Eng 2021, 12, 20417314211004816. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.T.; Hao, D.L.; Wu, B.N.; Mao, L.L.; Zhang, J. Uric acid demonstrates neuroprotective effect on Parkinson’s disease mice through Nrf2-ARE signaling pathway. Biochem. Biophys. Res. Commun. 2017, 493, 1443–1449. [Google Scholar] [CrossRef]
- Lv, Q.; Xu, D.; Zhang, X.; Yang, X.; Zhao, P.; Cui, X.; Liu, X.; Yang, W.; Yang, G.; Xing, S. Association of Hyperuricemia with Immune Disorders and Intestinal Barrier Dysfunction. Front. Physiol. 2020, 11, 524236. [Google Scholar] [CrossRef]
- Liu, X.; Lv, Q.; Ren, H.; Gao, L.; Zhao, P.; Yang, X.; Yang, G.; Xu, D.; Wang, G.; Yang, W.; et al. The altered gut microbiota of high-purine-induced hyperuricemia rats and its correlation with hyperuricemia. PeerJ 2020, 8, e8664. [Google Scholar] [CrossRef]
- Bakshi, R.; Macklin, E.A.; Logan, R.; Zorlu, M.M.; Xia, N.; Crotty, G.F.; Zhang, E.; Chen, X.; Ascherio, A.; Schwarzschild, M.A. Higher urate in LRRK2 mutation carriers resistant to Parkinson disease. Ann. Neurol. 2019, 85, 593–599. [Google Scholar] [CrossRef]
- Bougea, A.; Koros, C.; Papagiannakis, N.; Simitsi, A.M.; Prentakis, A.; Papadimitriou, D.; Pachi, I.; Antonelou, R.; Angelopoulou, E.; Beratis, I.; et al. Serum Uric Acid in LRRK2 Related Parkinson’s Disease: Longitudinal Data from the PPMI Study. J. Parkinsons Dis. 2021, 11, 633–640. [Google Scholar] [CrossRef]
- Koros, C.; Simitsi, A.M.; Papagiannakis, N.; Bougea, A.; Prentakis, A.; Papadimitriou, D.; Pachi, I.; Antonelou, R.; Angelopoulou, E.; Beratis, I.; et al. Serum uric acid level as a putative biomarker in Parkinson’s disease patients carrying GBA1 mutations: 2-Year data from the PPMI study. Parkinsonism Relat. Disord. 2021, 84, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, I.; Schlesinger, N. Uric acid in Parkinson’s disease. Mov. Disord. 2008, 23, 1653–1657. [Google Scholar] [CrossRef] [PubMed]
- Waring, W.S.; Webb, D.J.; Maxwell, S.R. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J. Cardiovasc. Pharmacol. 2001, 38, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Schwarzschild, M.A.; Macklin, E.A.; Bakshi, R.; Battacharyya, S.; Logan, R.; Espay, A.J.; Hung, A.Y.; Bwala, G.; Goetz, C.G.; Russell, D.S.; et al. Sex differences by design and outcome in the Safety of Urate Elevation in PD (SURE-PD) trial. Neurology 2019, 93, e1328–e1338. [Google Scholar] [CrossRef] [PubMed]
- Parkinson Study Group, S.-P.D.I.; Schwarzschild, M.A.; Ascherio, A.; Casaceli, C.; Curhan, G.C.; Fitzgerald, R.; Kamp, C.; Lungu, C.; Macklin, E.A.; Marek, K.; et al. Effect of Urate-Elevating Inosine on Early Parkinson Disease Progression: The SURE-PD3 Randomized Clinical Trial. JAMA 2021, 326, 926–939. [Google Scholar] [CrossRef]

| Characteristic | Result (n, %) |
|---|---|
| Sex (M: F) | 33 (67.3%): 16 (32.7%) |
| Mean age (years) | 69.1 ± 2.4, SD 8.6 |
| Age 65 years and older | 36 (73.5%) |
| Mean disease duration (years) | 5.1 ± 1.8, SD 5.2 |
| Hoehn and Yahr scale 1 | Stage ≤ 2.5: 22 (44.9%) Stage ≥ 3: 18 (36.7%) |
| Motor complications | 14 (28.6%) |
| Neurocognitive impairment | 16 (32.7%) |
| Cerebrovascular disease | 13 (26.5%) |
| Mean daily levodopa equivalent dose (mg) | 925.2 ± 178.2, SD 603.1 |
| Associations of Lower UA Levels | Correlation Coefficient | p-Value |
|---|---|---|
| Severity of parkinsonism (Hoehn and Yahr scale) | rs = 0.488 | 0.002 |
| Motor complications | r = 0.333 | 0.027 |
| Neurocognitive impairment | r = 0.346 | 0.021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dănău, A.; Dumitrescu, L.; Lefter, A.; Popescu, B.O. Serum Uric Acid Levels in Parkinson’s Disease: A Cross-Sectional Electronic Medical Record Database Study from a Tertiary Referral Centre in Romania. Medicina 2022, 58, 245. https://doi.org/10.3390/medicina58020245
Dănău A, Dumitrescu L, Lefter A, Popescu BO. Serum Uric Acid Levels in Parkinson’s Disease: A Cross-Sectional Electronic Medical Record Database Study from a Tertiary Referral Centre in Romania. Medicina. 2022; 58(2):245. https://doi.org/10.3390/medicina58020245
Chicago/Turabian StyleDănău, Adela, Laura Dumitrescu, Antonia Lefter, and Bogdan Ovidiu Popescu. 2022. "Serum Uric Acid Levels in Parkinson’s Disease: A Cross-Sectional Electronic Medical Record Database Study from a Tertiary Referral Centre in Romania" Medicina 58, no. 2: 245. https://doi.org/10.3390/medicina58020245






