Analysis of Single-Leg Hopping in Long-Term Treated Patients with Neurological Wilson’s Disease: A Controlled Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Neurological Examination of Patients and Clinical Scores
2.3. Laboratory Findings
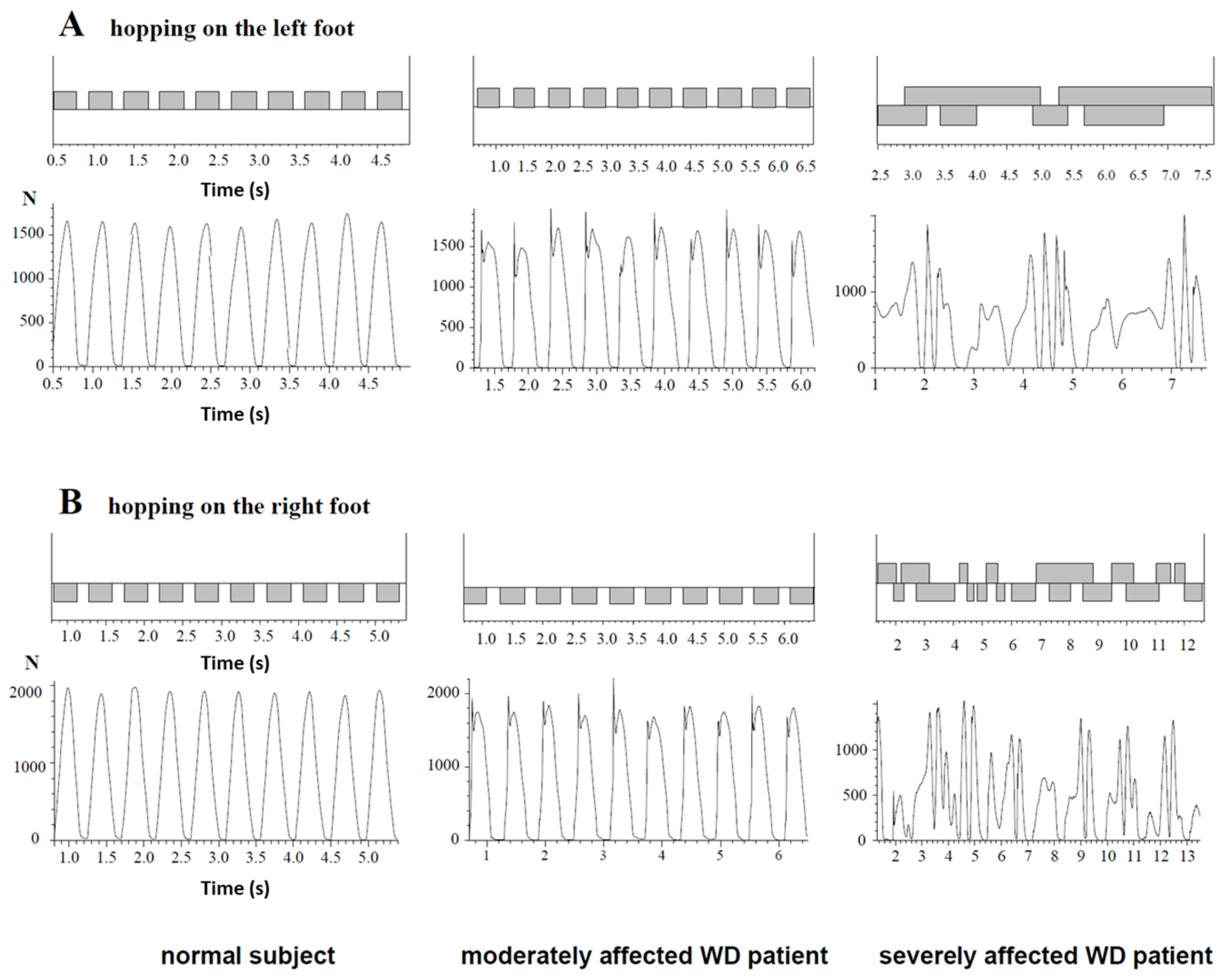
2.4. Measurement of Hopping by Means of the Infotronic® Gait Analysis System
2.5. Statistics
3. Results
3.1. Comparison of the WD-Patients and the Control Subjects
3.2. Treatment-Related Data and Clinical Examination of the WD-Patients
3.3. Ability to Perform Single-Leg Hopping
3.4. Comparison of the NO-HOP- and the HOP-Subgroup
3.5. Comparison of Single-Leg Hopping in Patients with WD and Controls
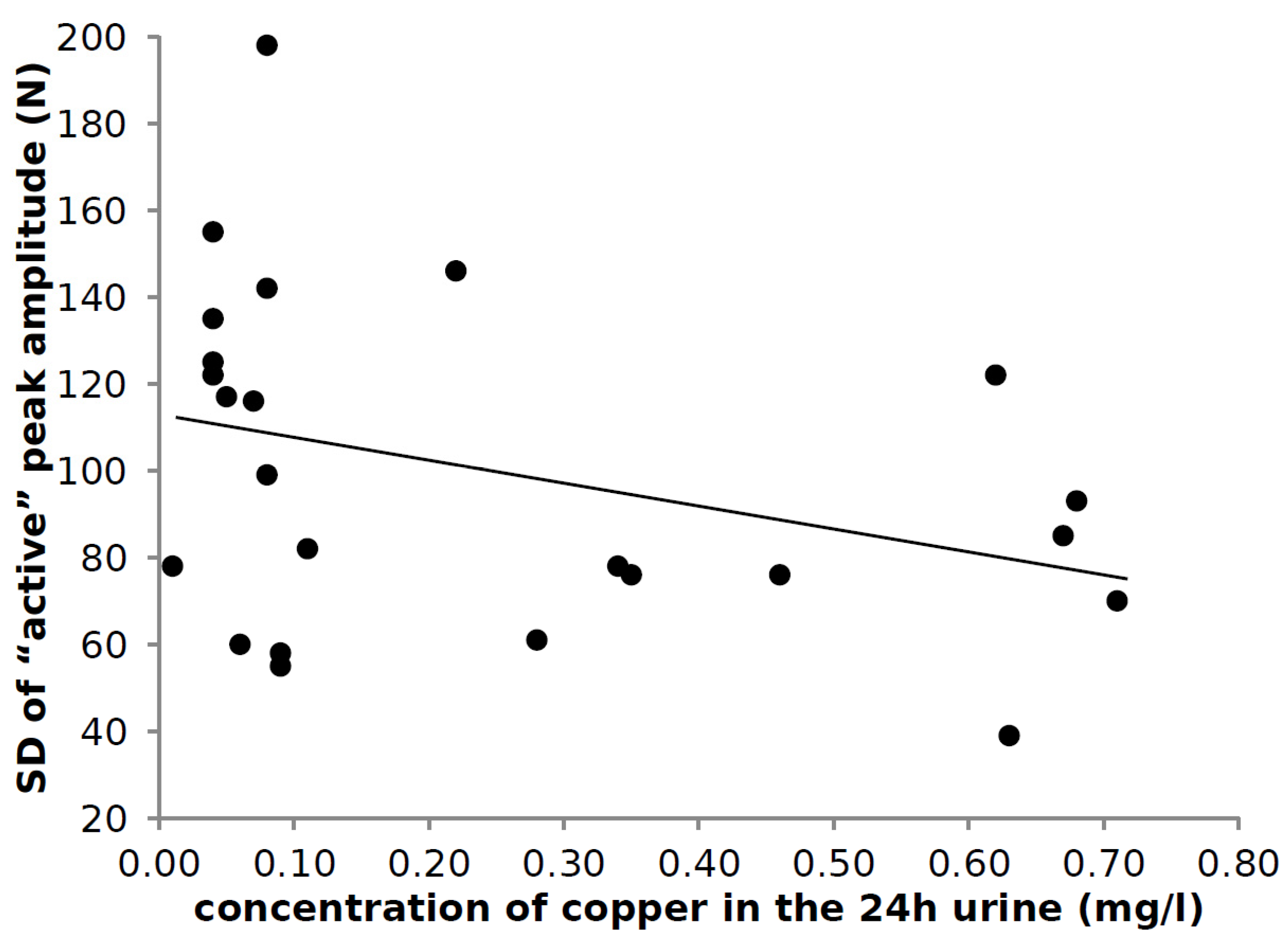
3.6. Correlation of Laboratory Findings and Parameters of Hopping
4. Discussion
4.1. Learning to Hop and the Age of Onset of Symptoms in WD
4.2. Response to Therapy
4.3. High Percentage of Patients with an Initial “Impact” Peak
4.4. Analysis of Hopping Frequency in WD
4.5. Variability of the Peak Amplitude
5. Conclusions
Strengths and Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bull, P.; Thomas, G.R.; Rommens, J.M.; Forbes, J.R.; Cox, D.W. The Wilson disease gene is a putative copper transporting P–type ATPase similar to the Menkes gene. Nat. Genet. 1993, 5, 327–337. [Google Scholar] [CrossRef]
- Petrukhin, K.; Fischer, S.G.; Pirastu, M.; Tanzi, R.E.; Chernov, I.; Devoto, M.; Brzustowicz, L.M.; Cayanis, E.; Vitale, E.; Russo, J.J.; et al. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat. Genet. 1993, 5, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E.; Petrukhin, K.; Chernov, I.; Pellequer, J.-L.; Wasco, W.; Ross, B.; Romano, D.M.; Parano, E.; Pavone, L.; Brzustowicz, L.M.; et al. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat. Genet. 1993, 5, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Cumings, J.N. The copper and iron content of brain and liver in the normal and in hepato-lenticular degeneration. Brain 1948, 71, 410–415. [Google Scholar] [CrossRef]
- Kuwert, T.; Hefter, H.; Scholz, D.; Milz, M.; Arendt, G.; Herzog, H.; Loken, M.; Hennerici, M.; Feinendegen, L.E. Regional cerebral glucose consumption measured by positron emission tomography in patients with Wilson’s disease. Eur. J. Pediatr. 1992, 19, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.F.; Dayalu, P.; Nan, B.; Askari, F.; Brewer, G.J.; Lorincz, M.T. Prognostic significance of neurologic examination findings in Wilson disease. Parkinsonism Relat. Disord. 2011, 17, 551–556. [Google Scholar] [CrossRef]
- Oder, W.; Grimm, G.; Kollegger, H.; Ferenci, P.; Schneider, B.; Deecke, L. Neurological and neuropsychiatric spectrum of Wilson’s disease: A prospective study of 45 cases. J. Neurol. 1991, 238, 281–287. [Google Scholar]
- Stremmel, W.; Meyerrose, K.-W.; Niederau, C.; Hefter, H.; Kreuzpaintner, G.; Strohmeyer, G. Wilson Disease: Clinical Presentation, Treatment, and Survival. Ann. Intern. Med. 1991, 115, 720. [Google Scholar] [CrossRef]
- Machado, A.; Chien, H.F.; Deguti, M.M.; Cancado, E.; Azevedo, R.; Scaff, M.; Barbosa, E.R. Neurological manifestations in Wilson’s disease: Report of 119 cases. Mov. Disord. 2006, 21, 2192–2196. [Google Scholar] [CrossRef]
- Taly, A.B.; Meenakshi-Sundaram, S.; Sinha, S.; Swamy, H.S.; Arunodaya, G.R. Wilson disease: Description of 282 patients evaluated over 3 decades. Medicine 2007, 86, 112–121. [Google Scholar] [CrossRef]
- Dziezyc, K.; Litwin, T.; Chabik, G.; Czlonkowska, A. Frequencies of initial gait disturbances and falls in 100 Wilson’s disease patients. Gait Posture 2015, 42, 601–603. [Google Scholar] [CrossRef]
- Giagheddu, A.; Demelia, L.; Puggioni, G.; Nurchi, A.M.; Contu, L.; Pirari, G.; Deplano, A.; Rachele, M.G. Epidemiologic study of hepatolenticular degeneration (Wilson’s disease) in Sardinia (1902–1983). Acta Neurol. Scand. 1985, 72, 43–55. [Google Scholar] [CrossRef]
- Hefter, H.; Arendt, G.; Kuwert, T.; Herzog, H.; Feinendegen, L.E.; Stremmel, W. Relationship between striatal glucose consumption and copper excretion in patients with Wilson’s disease treated with D-penicillamine. J. Neurol. 1993, 241, 49–53. [Google Scholar] [CrossRef]
- Hefter, H.; Tezayak, O.; Rosenthal, D. Long-term outcome of neurological Wilson’s disease. Parkinsonism Relat. Disord. 2018, 49, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Tezayak, O.; Rosenthal, D.; Hefter, H. Mild gait impairment in long-term treated patients with neurological Wilson’s disease. Ann. Transl. Med. 2019, 7, S57. [Google Scholar] [CrossRef] [PubMed]
- Samadzadeh, S.; Hefter, H.; Tezayak, O.; Rosenthal, D. Mildly Impaired Foot Control in Long-Term Treated Patients with Wilson’s Disease. J. Funct. Morphol. Kinesiol. 2022, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Halverson, L.; Williams, K. Developmental Sequences for Hopping over Distance: A Prelongitudinal Screening. Res. Q. Exerc. Sport 1985, 56, 37–44. [Google Scholar] [CrossRef]
- Holm, I.; Tveter, A.T.; Fredriksen, P.M.; Vøllestad, N. A normative sample of gait and hopping on one leg parameters in children 7–12 years of age. Gait Posture 2009, 29, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Whitall, J.; Getchell, N. From walking to running: Applying a dynamical systems approach to the development of locomotor skills. Child Dev. 1995, 66, 1541–1553. [Google Scholar] [CrossRef]
- Woollacott, M.H.; Shumway-Cook, A. Development of Posture and Gait across the Life Span; University of South Carolina Press: Columbia, SC, USA, 1989. [Google Scholar]
- de Kegel, A.; Dhooge, I.; Peersman, W.; Rijckaert, J.; Baetens, T.; Cambier, D.; van Waelvelde, H. Construct validity of the assessment of balance in children who are developing typically and in children with hearing impairment. Phys. Ther. 2010, 90, 1783–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.; Cockcroft, J.; Louw, Q.; Brink, Y. Single leg hopping in children with fetal alcohol spectrum disorder: Dynamic postural stability and kinematics. J. Bodyw. Mov. Ther. 2020, 24, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Beerse, M.; Wu, J. Vertical stiffness and balance control of two-legged hopping in-place in children with and without Down syndrome. Gait Posture 2018, 63, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Verbecque, E.; da Costa, P.H.L.; Vereeck, L.; Hallemans, A. Psychometric properties of functional balance tests in children: A literature review. Dev. Med. Child Neurol. 2014, 57, 521–529. [Google Scholar] [CrossRef]
- Cavagna, G.A.; Franzetti, P.; Heglund, N.C.; Willems, P. The determinants of the step frequency in running, trotting and hopping in man and other vertebrates. J. Physiol. 1988, 399, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Blickhan, R. The spring-mass model for running and hopping. J. Biomech. 1989, 22, 1217–1227. [Google Scholar] [CrossRef]
- Schepens, B.; Willems, P.A.; Caravagna, G.A. The mechanics of running in children. J. Physiol. 1998, 509, 927–940. [Google Scholar] [CrossRef]
- Hefter, H.; Arendt, G.; Stremmel, W.; Freund, H.-J. Motor impairment in Wilson’s disease, I: Slowness of voluntary limb movements. Acta Neurol. Scand. 1993, 87, 133–147. [Google Scholar] [CrossRef]
- Hefter, H.; Arendt, G.; Stremmel, W.; Freund, H.-J. Motor impairment in Wilson’s disease, II: Slowness of speech. Acta Neurol. Scand. 1993, 87, 148–160. [Google Scholar] [CrossRef]
- Medici, V.; Mirante, V.G.; Fassati, L.R.; Pompili, M.; Forti, D.; del Gaudio, M.; Trevisan, C.P.; Cillo, U.; Sturniolo, G.C.; Fagiuoli, S. Liver transplantation for Wilson’s disease: The burden of neurological and psychiatric disorders. Liver Transplant. 2005, 11, 1056–1063. [Google Scholar] [CrossRef]
- Available online: https:///www.researchgate.net/figure/The-shoes-Computer-Dyno-Graphy-CDGR-system-Infotronic-Netherlands-Shown-is-the_fig1_23681105 (accessed on 15 November 2021).
- Ghika, J.; Vingerhoets, F.; Maeder, P.; Borruat, F.-X.; Bogousslavsky, J. Maladie de Wilson. EMC Neurol. 2004, 1, 481–511. [Google Scholar] [CrossRef]
- Barnes, N.; Tsivkovskii, R.; Tsivkovskaia, N.; Lutsenko, S. The Copper-transporting ATPases, Menkes and Wilson Disease Proteins, Have Distinct Roles in Adult and Developing Cerebellum. J. Biol. Chem. 2005, 280, 9640–9645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erfanizadeh, M.; Noorafshan, A.; Naseh, M.; Karbalay-Doust, S. The effects of copper sulfate on the structure and function of the rat cerebellum: A stereological and behavioral study. IBRO Neurosci. Rep. 2021, 11, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Min, J.O.; Kang, D.-S.; Kim, Y.S.; Jung, G.H.; Park, H.J.; Kim, S.; An, H.; Kwon, J.; Kim, J. Control of motor coordination by astrocytic tonic GABA release through modulation of excitation/inhibition balance in cerebellum. Proc. Natl. Acad. Sci. USA 2018, 115, 5004–5009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGee, T.P.; Houston, C.M.; Brickley, S.G. Copper Block of Extrasynaptic GABAA Receptors in the Mature Cerebellum and Striatum. J. Neurosci. 2013, 33, 13431–13435. [Google Scholar] [CrossRef] [Green Version]
- Popescu, B.F.G.; Robinson, C.A.; Rajput, A.; Rajput, A.H.; Harder, S.L.; Nichol, H. Iron, copper, and zinc distribution of the cerebellum. Cerebellum 2009, 8, 74–79. [Google Scholar] [CrossRef]
- Zuur, A.T.; Lundbye-Jensen, J.; Leukel, C.; Taube, W.; Grey, M.J.; Gollhofer, A.; Nielsen, J.B.; Grüber, M. Contribution of afferent feedback and descending drive to human hopping. J. Physiol. 2010, 588, 799–807. [Google Scholar] [CrossRef]
- Liu, W.; Nigg, B.M. A mechanical model to determine the influence of masses and mass distribution on the impact force during running. J. Biomech. 2000, 33, 219–224. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Nikooyan, A.A.; Arshi, A.R. A model-based parametric study of impact force during running. J. Biomech. 2007, 40, 2012–2021. [Google Scholar] [CrossRef]
- Crossley, K.; Bennell, K.L.; Wrigley, T.; Oakes, B.W. Ground reaction forces, bone characteristics, and tibial stress fracture in male runners. Med. Sci. Sports Exerc. 1999, 31, 1088–1093. [Google Scholar] [CrossRef]
- Bennell, K.; Crossley, K.; Jayarajan, J.; Walton, E.; Warden, S.; Kiss, Z.S.; Wrigley, T. Ground reaction forces and bone parameters in females with tibial stress fracture. Med. Sci. Sports Exerc. 2004, 36, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Creaby, M.W.; Allsopp, A.J. Comparison of static and dynamic biomechanical measures in military recruits with and without a history of third metatarsal stress fracture. Clin. Biomech. 2005, 21, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Divert, C.; Mornieux, G.; Baur, H.; Mayer, F.; Belli, A. Mechanical Comparison of Barefoot and Shod Running. Int. J. Sports Med. 2005, 26, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Wheat, J.; Barlett, R.M.; Milner, C.E.; Mullineaux, D.R. The effect of different surfaces on ground reaction forces during running: A single-individual design approach. J. Hum. Mov. Stud. 2003, 44, 353–364. [Google Scholar]
- Nikooyan, A.A.; Zadpoor, A.A. Mass–spring–damper modelling of the human body to study running and hopping–An overview. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2011, 225, 1121–1135. [Google Scholar] [CrossRef]
- Nigg, B.M.; Liu, W. The effect of muscle stiffness and damping on simulated impact force peaks during running. J. Biomech. 1999, 32, 849–856. [Google Scholar] [CrossRef]
- Beerse, M.; Wu, J. Lower Limb Joint Functions during Single-Leg Hopping in-Place in Children and Adults. J. Mot. Behav. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Alexander, R.M. Hopping and running on two legs or four. Nature 1980, 287, 187. [Google Scholar] [CrossRef]
- Caravagna, G.A.; Legramandi, M.A. The bounce of the body in hopping, running and trotting: Different machines with the same motor. Proc. R. Soc. B 2009, 276, 4279–4285. [Google Scholar] [CrossRef] [Green Version]
- Samadzadeh, S.; Hefter, H.; Tezayak, O.; Rosenthal, D. Analysis of Running in Wilson’s Disease. Sports 2022, 10, 11. [Google Scholar] [CrossRef]
- Haeufle, D.F.B.; Grimmer, S.; Kalveram, K.-T.; Seyfarth, A. Integration of intrinsic muscle properties, feed-forward and feedback signals for generating and stabilizing hopping. J. R. Soc. Interface 2012, 9, 1458–1469. [Google Scholar] [CrossRef] [Green Version]
- Volkmann, J.; Hefter, H.; Lange, H.W.; Freund, H.-J. Impairment of temporal organization of speech in basal ganglia diseases. Brain Lang. 1992, 43, 386–399. [Google Scholar] [CrossRef]
- Dusek, P.; Litwin, T.; Członkowska, A. Neurologic impairment in Wilson disease. Ann. Transl. Med. 2019, 7, S64. [Google Scholar] [CrossRef] [PubMed]
- Socha, P.; Czlonkowska, A.; Janczyk, W.; Litwin, T. Wilson’s disease-management and long-term outcomes. Best Pract. Res. Clin. Gastroenterol. 2021; in press. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef] [Green Version]


| Part A | |||||
| Parameter | WD-Patients n = 30 | Control Subjects n = 30 | p-Value | ||
| Age (years) | mean (SD) | 34.2 (11.0) | 32.8 (8.3) | p = 0.571; n.s. | |
| range | 15–56 | 14–51 | |||
| Sex | m/f | 19/11 | 19/11 | p = 1.000; n.s. | |
| Body height (cm) BH | mean (SD) | 177.8 (10.3) | 176.9 (9.5) | p = 0.730; n.s. | |
| range | 156–196 | 160–194 | |||
| Body weight (kg) BW | mean (SD) | 72.7 (1.5) | 73.6 (14.8) | p = 0.830; n.s. | |
| range | 48–126 | 51–105 | |||
| Part B | |||||
| Parameter | HOP-Group n = 24 | Control Subjects n = 30 | p-Value | ||
| HF (1/s) | L | mean (SD) | 1.94 (0.29) | 1.95 (0.18) | p = 0.681; n.s. |
| R | mean (SD) | 1.90 (0.33) | 1.92 (0.18) | p = 0.841; n.s. | |
| DFC (ms) | L | mean (SD) | 373 (94) | 368 (50) | p = 0.823; n.s. |
| R | mean (SD) | 362 (90) | 357 (46) | p = 0.675; n.s. | |
| PA/BW (N/kg) | L | mean (SD) | 23.25 (4.95) | 24.85 (3.20) | p = 0.159; n.s. |
| R | mean (SD) | 23.12 (4.82) | 25.04 (3.02) | p = 0.080; n.s. | |
| PT (ms) | L | mean (SD) | 127.8 (27.2) | 144.4 (24.6) | p < 0.022 |
| R | mean (SD) | 126.9 (30.2) | 146.8 (27.5) | p < 0.014 | |
| Part A | ||||
| Parameter | NO-HOP Subgroup (n = 5) | HOP Subgroup (n = 24) | Level of Significance | |
| MIL-group | number of pats. | 0 | 10 | |
| MOD-group | number of pats. | 1 | 10 | |
| SEV-group | number of pats. | 4 | 4 | p < 0.002 |
| Part B | ||||
| Parameter | ||||
| N-MotS = 0 | number of pats. | 0 | 19 | |
| N-MotS > 0 | number of pats. | 5 | 5 | p < 0.002 |
| Part C | ||||
| No psychiatric symptoms | number of pats. | 0 | 21 | |
| With psychiatric symptoms | number of pats. | 5 | 3 | p < 0.001 |
| Part D | ||||
| TSC | MV/SD | 10.8/3.6 | 3.4/2.4 | p < 0.0001 |
| MotS | MV/SD | 7.4/3.0 | 3.0/2.0 | p < 0.0001 |
| N-MotS | MV/SD | 3.4/0.9 | 0.4/0.9 | p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hefter, H.; Samadzadeh, S.; Rosenthal, D.; Tezayak, O. Analysis of Single-Leg Hopping in Long-Term Treated Patients with Neurological Wilson’s Disease: A Controlled Pilot Study. Medicina 2022, 58, 249. https://doi.org/10.3390/medicina58020249
Hefter H, Samadzadeh S, Rosenthal D, Tezayak O. Analysis of Single-Leg Hopping in Long-Term Treated Patients with Neurological Wilson’s Disease: A Controlled Pilot Study. Medicina. 2022; 58(2):249. https://doi.org/10.3390/medicina58020249
Chicago/Turabian StyleHefter, Harald, Sara Samadzadeh, Dietmar Rosenthal, and Osman Tezayak. 2022. "Analysis of Single-Leg Hopping in Long-Term Treated Patients with Neurological Wilson’s Disease: A Controlled Pilot Study" Medicina 58, no. 2: 249. https://doi.org/10.3390/medicina58020249






