Non-Marked Hypoechogenic Nodules: Multicenter Study on the Thyroid Malignancy Risk Stratification and Accuracy Based on TIRADS Systems Comparison
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Acquisition of Data
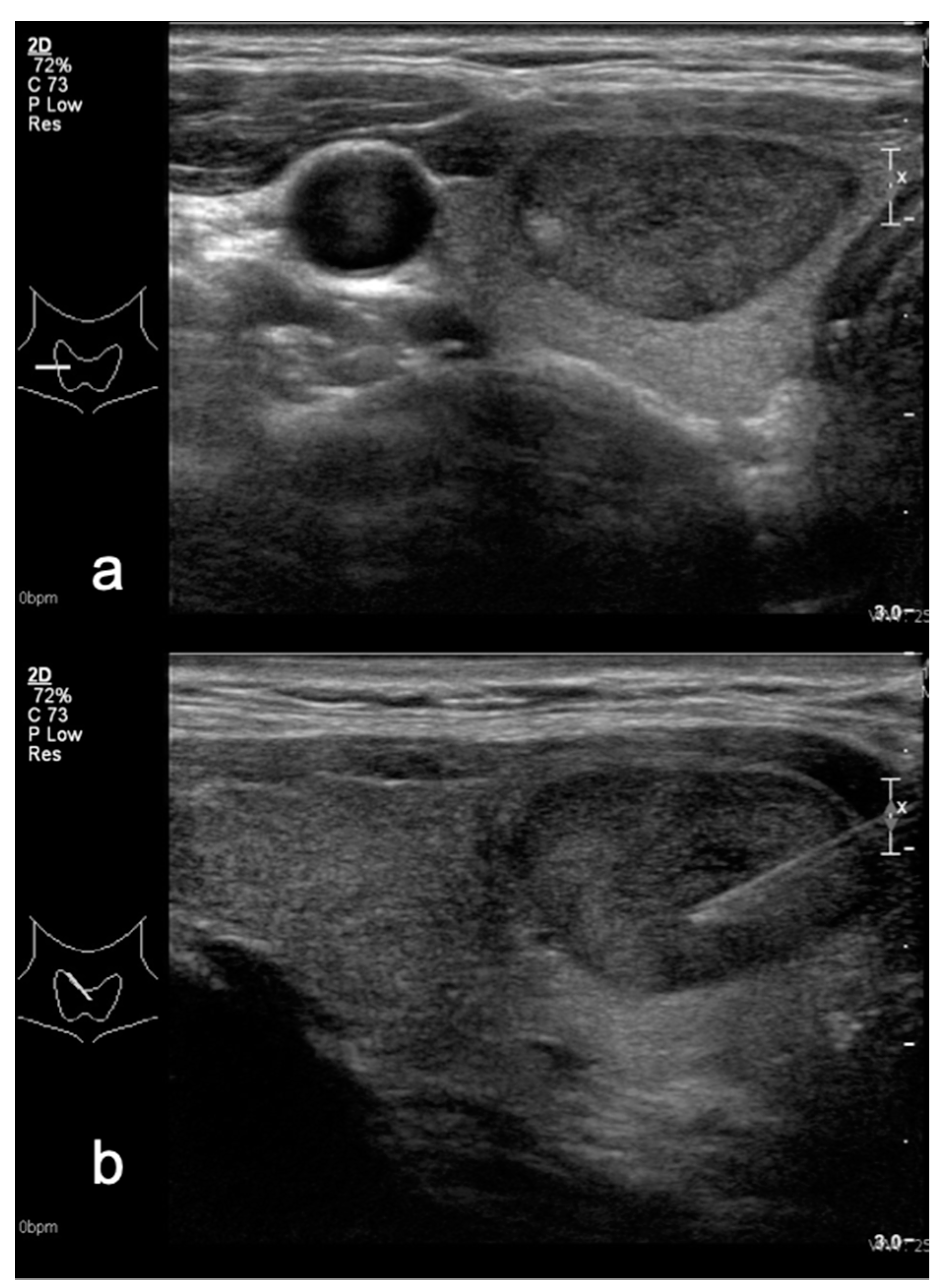
2.3. US Examination
2.4. Modified TIRADS Classification
2.5. Fine Needle Aspiration (FNA)
2.6. Standard of Reference
2.7. Statistical Analysis
3. Results
3.1. Performance of M-TIRADS and K-TIRADS Using FNA as Reference Standard
3.2. Results of M-TIRADS in the Subgroup of Surgically Treated Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib, H.; Papini, E.; Paschke, R.; Duick, D.S.; Valcavi, R.; Hegedüs, L.; Vitti, P.; Tseleni-Balafouta, S.; Baloch, Z.; Crescenzi, A.; et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: Executive summary of recommendations. Endocr. Pract. 2010, 16, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Solbiati, L.; Charboneau, J.W.; Cantisani, V.; Reading, C.; Mauri, G. The Thyroid Gland.Diagnostic Ultrasound, 5th ed.; Elsevier: Philadelphia, PA, USA, 2018; pp. 691–731. Available online: https://www.clinicalkey.com/#!/content/book/3-s2.0-B9780323401715000195?scrollTo=%23hl0001081 (accessed on 24 August 2021).
- Basha, M.A.A.; Alnaggar, A.A.; Refaat, R.; Maghraby, A.M.E.; Refaat, M.M.; Elhamed, M.E.A.; Abdalla, A.E.; Aly, S.A.; Hanafy, A.S.; Mohamed, A.E.M.; et al. The validity and reproducibility of the thyroid imaging reporting and data system (TI-RADS) in categorization of thyroid nodules: Multicentre prospective study. Eur. J. Radiol. 2019, 117, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Uruno, T.; Nakano, K.; Takamura, Y.; Miya, A.; Kobayashi, K.; Yokozawa, T.; Matsuzuka, F.; Kuma, S.; Kuma, K.; et al. An observation trial without surgical treatment in patients with papillary microcarcinoma of the thyroid. Thyroid 2003, 13, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Ito, Y.; Miyauchi, A.; Inoue, H.; Fukushima, M.; Kihara, M.; Higashiyama, T.; Tomoda, C.; Takamura, Y.; Kobayashi, K.; Miya, A. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J. Surg. 2010, 34, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Miyauchi, A.; Ito, Y.; Oda, H. Insights into the Management of Papillary Microcarcinoma of the Thyroid. Thyroid 2017, 28, 23–31. [Google Scholar] [CrossRef]
- Yang, R.; Zou, X.; Zeng, H.; Zhao, Y.; Ma, X. Comparison of Diagnostic Performance of Five Different Ultrasound TI-RADS Classification Guidelines for Thyroid Nodules. Front. Oncol. 2020, 10, 598225. [Google Scholar] [CrossRef]
- Horvath, E.; Majlis, S.; Rossi, R.; Franco, C.; Niedmann, J.P.; Castro, A.; Dominguez, M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J. Clin. Endocrinol. Metab. 2009, 94, 1748–1751. [Google Scholar] [CrossRef]
- Grani, G.; D’Alessandri, M.; Carbotta, G.; Nesca, A.; Del Sordo, M.; Alessandrini, S.; Coccaro, C.; Rendina, R.; Bianchini, M.; Prinzi, N.; et al. Grey-Scale Analysis Improves the Ultrasonographic Evaluation of Thyroid Nodules. Medicine 2015, 94, e1129. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, S.M.; Choi, S.I. Diagnostic accuracy of ultrasound features in thyroid microcarcinomas. Endocr. J. 2008, 55, 931–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, J.Y.; Han, K.H.; Yoon, J.H.; Moon, H.J.; Son, E.J.; Park, S.H.; Jung, H.K.; Choi, J.S.; Kim, B.M.; Kim, E.K. Thyroid imaging reporting and data system for US features of nodules: A step in establishing better stratification of cancer risk. Radiology 2011, 260, 892–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Na, D.G.; Yoon, S.J.; Gwon, H.Y.; Paik, W.; Kim, T.; Kim, J.Y. Ultrasound malignancy risk stratification of thyroid nodules based on the degree of hypoechogenicity and echotexture. Eur. Radiol. 2020, 30, 1653–1663. [Google Scholar] [CrossRef] [PubMed]
- Bonavita, J.A.; Mayo, J.; Babb, J.; Bennett, G.; Oweity, T.; Macari, M.; Yee, J. Pattern recognition of benign nodules at ultrasound of the thyroid: Which nodules can be left alone? AJR Am. J. Roentgenol. 2009, 193, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Chen, C.N.; Chen, K.Y.; Ho, M.C.; Tai, H.C.; Wang, Y.H.; Chen, A.; Chang, K.J. Quantitative analysis of echogenicity for patients with thyroid nodules. Sci. Rep. 2016, 6, 35632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Yousef, M.M.; Larson, J.H.; Kuehn, D.M.; Wu, A.S.; Laroia, A.T. Safety of ultrasound-guided fine needle aspiration biopsy of neck lesions in patients taking antithrombotic/anticoagulant medications. Ultrasound Q. 2011, 27, 157–159. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The 2017 Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2017, 27, 1341–1346. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The Bethesda system for reporting thyroid cytopathology. Am. J. Clin. Pathol. 2009, 132, 658–665. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.-H.; Lee, Y.H.; Lim, H.K.; Moon, W.-J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [Green Version]
- Russ, G.; Royer, B.; Bigorgne, C.; Rouxel, A.; Bienvenu-Perrard, M.; Leenhardt, L. Prospective evaluation of thyroid imaging reporting and data system on 4550 nodules with and without elastography. Eur. J. Endocrinol. 2013, 168, 649–655. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid J. 2017, 6, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Han, K.; Kim, E.K.; Moon, H.J.; Kwak, J.Y. Diagnosis and Management of Small Thyroid Nodules: A Comparative Study with Six Guidelines for Thyroid Nodules. Radiology 2017, 283, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, E.J.; Moon, W.J.; Na, D.G.; Lee, Y.H.; Choi, N.; Kim, S.J.; Kim, J.K. A Multicenter Prospective Validation Study for the Korean Thyroid Imaging Reporting and Data System in Patients with Thyroid Nodules. Korean J. Radiol. 2016, 17, 811–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nattabi, H.A.; Sharif, N.M.; Yahya, N.; Ahmad, R.; Mohamad, M.; Zaki, F.M.; Yusoff, A.N. Is Diagnostic Performance of Quantitative 2D-Shear Wave Elastography Optimal for Clinical Classification of Benign and Malignant Thyroid Nodules?: A Systematic Review and Meta-Analysis. Acad. Radiol. 2017, in press. [Google Scholar] [CrossRef]
- Cosgrove, D.; Barr, R.; Bojunga, J.; Cantisani, V.; Chammas, M.C.; Dighe, M.; Vinayak, S.; Xu, J.-M.; Dietrich, C.F. WFUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography: Part 4. Thyroid. Ultrasound Med. Biol. 2017, 43, 4–26. [Google Scholar] [CrossRef]
- Magri, F.; Chytiris, S.; Croce, L.; Molteni, M.; Bendotti, G.; Gruosso, G.; Tata Ngnitejeu, S.; Agozzino, M.; Rotondi, M.; Chiovato, L. Performance of the ACR TI-RADS and EU TI-RADS scoring systems in the diagnostic work-up of thyroid nodules in a real-life series using histology as reference standard. Eur. J. Endocrinol. 2020, 183, 521–528. [Google Scholar] [CrossRef]
- Kim, E.K.; Park, C.S.; Chung, W.Y.; Oh, K.K.; Kim, D.I.; Lee, J.T.; Yoo, H.S. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am. J. Roentgenol. 2002, 178, 687–691. [Google Scholar] [CrossRef] [Green Version]
- Na, D.G.; Baek, J.H.; Sung, J.Y.; Kim, J.-H.; Kim, J.K.; Choi, Y.J.; Seo, H. Thyroid Imaging Reporting and Data System Risk Stratification of Thyroid Nodules: Categorization Based on Solidity and Echogenicity. Thyroid 2016, 26, 562–572. [Google Scholar] [CrossRef]
- Grani, G.; Lamartina, L.; Cantisani, V.; Maranghi, M.; Lucia, P.; Durante, C. Interobserver agreement of various thyroid imaging reporting and data systems. Endocr. Connect. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef] [Green Version]





| US Feature | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR |
|---|---|---|---|---|---|
| Marked hypoechogenicity | 64.86 | 94.32 | 45.28 | 97.37 | 11.43 |
| Microcalcification | 67.57 | 90.02 | 32.89 | 97.46 | 6.77 |
| Irregular margins | 75.68 | 93.74 | 46.67 | 98.16 | 12.09 |
| AP > LL | 29.73 | 98.63 | 61.11 | 95.05 | 21.07 |
| Lymphadenopathy | 19.22 | 100 | 100 | 94.28 | 177.32 |
| M-TIRADS | 93.02 (80.94–98.54) | 81.31 (77.69–84.57) | 29.2 (21.75–37.57) | 99.29 (97.95–99.85) | 18.62 |
| Categories | Based on TIRADS by Kwak (n = 424) | Based on M-TIRADS (n = 460) |
|---|---|---|
| TIRADS categories | - | - |
| TIRADS 3 benign/malignant | 172/1 | 320/3 |
| TIRADS 4A benign/malignant | 168/2 | 72/5 |
| TIRADS 4B benign/malignant | 42/11 | 20/12 |
| TIRADS 4C benign/malignant | 9/7 | - |
| TIRADS 5 benign/malignant | 0/12 | 5/23 |
| Risk of malignancy | - | - |
| TIRADS 2 | 0 | 0 |
| TIRADS 3 | 0.4 | 0.9 |
| TIRADS 4A | 0.8 | 6.5 |
| TIRADS 4B | 11.7 | 37.5 |
| TIRADS 4C | 23.3 | - |
| TIRADS 5 | 75 | 82.1 |
| M-TIRADS | Cytology | Histology | ||
|---|---|---|---|---|
| Bethesda Category | Cases | Category | Cases | |
| M-TIRADS 2 | Benign | 7 | Benign | 6 |
| Lymphoma | 1 | |||
| M-TIRADS 3 | Nondiagnostic | 7 | Benign | 7 |
| Benign | 25 | Benign | 22 | |
| Thyroiditis | 1 | |||
| Follicular carcinoma | 2 | |||
| Follicular pathology | 4 | Benign | 2 | |
| Papillary carcinoma | 2 | |||
| Follicular neoplasia | 1 | Benign | 1 | |
| Suspicious | 3 | Papillary carcinoma | 2 | |
| Thyroiditis | 1 | |||
| Malignant | 1 | Papillary carcinoma | 1 | |
| No cytology | 3 | Benign | 3 | |
| M-TIRADS 4A | Nondiagnostic | 3 | Benign | 2 |
| Papillary carcinoma | 1 | |||
| Benign | 5 | Benign | 5 | |
| Follicular pathology | 1 | Benign | 1 | |
| Follicular neoplasia | 1 | Papillary carcinoma | 1 | |
| Suspicious | 5 | Benign | 1 | |
| Papillary carcinoma | 3 | |||
| Medullar carcinoma | 1 | |||
| Malignant | 4 | Benign | 2 | |
| Papillary carcinoma | 2 | |||
| M-TIRADS 4B | Nondiagnostic | 2 | Benign | 2 |
| Benign | 2 | Benign | 2 | |
| Follicular pathology | 1 | Benign | 1 | |
| Follicular neoplasia | 2 | Papillary carcinoma | 1 | |
| Follicular carcinoma | 1 | |||
| Suspicious | 6 | Benign | 3 | |
| Papillary carcinoma | 2 | |||
| Thyroiditis | 1 | |||
| Malignant | 10 | Papillary carcinoma | 10 | |
| No cytology | 1 | Benign | 1 | |
| M-TIRADS 5 | Nondiagnostic | 2 | Benign | 1 |
| Anaplastic carcinoma | 1 | |||
| Benign | 2 | Benign | 2 | |
| Suspicious | 5 | Papillary carcinoma | 4 | |
| Thyroiditis | 1 | |||
| Malignant | 15 | Benign | 2 | |
| Papillary carcinoma | 10 | |||
| Medullar carcinoma | 3 | |||
| No cytology | 1 | Papillary carcinoma | 1 | |
| Nr. | Bethesda Category | US Echogenicity | Histology Result |
|---|---|---|---|
| 1. | Follicular pathology | Hypo | Papillary carcinoma (follicular) |
| 2. | Suspicious | Hypo | Papillary carcinoma |
| 3. | Malignant | Hypo | Papillary carcinoma (follicular), multifocal |
| 4. | Benign | Iso | Microfollicular carcinoma |
| 5. | Benign | Iso | Papillary carcinoma (follicular) |
| 6. | Benign | Iso | Microfollicular carcinoma |
| 7. | Not known | Iso | Papillary carcinoma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prieditis, P.; Radzina, M.; Mikijanska, M.; Liepa, M.; Stepanovs, K.; Grani, G.; Durante, C.; Lamartina, L.; Trimboli, P.; Cantisani, V. Non-Marked Hypoechogenic Nodules: Multicenter Study on the Thyroid Malignancy Risk Stratification and Accuracy Based on TIRADS Systems Comparison. Medicina 2022, 58, 257. https://doi.org/10.3390/medicina58020257
Prieditis P, Radzina M, Mikijanska M, Liepa M, Stepanovs K, Grani G, Durante C, Lamartina L, Trimboli P, Cantisani V. Non-Marked Hypoechogenic Nodules: Multicenter Study on the Thyroid Malignancy Risk Stratification and Accuracy Based on TIRADS Systems Comparison. Medicina. 2022; 58(2):257. https://doi.org/10.3390/medicina58020257
Chicago/Turabian StylePrieditis, Peteris, Maija Radzina, Madara Mikijanska, Mara Liepa, Kaspars Stepanovs, Giorgio Grani, Cosimo Durante, Livia Lamartina, Pierpaolo Trimboli, and Vito Cantisani. 2022. "Non-Marked Hypoechogenic Nodules: Multicenter Study on the Thyroid Malignancy Risk Stratification and Accuracy Based on TIRADS Systems Comparison" Medicina 58, no. 2: 257. https://doi.org/10.3390/medicina58020257






