The Usefulness of Serological Inflammatory Markers in Patients with Rotator Cuff Disease—A Systematic Review
Abstract
1. Introduction
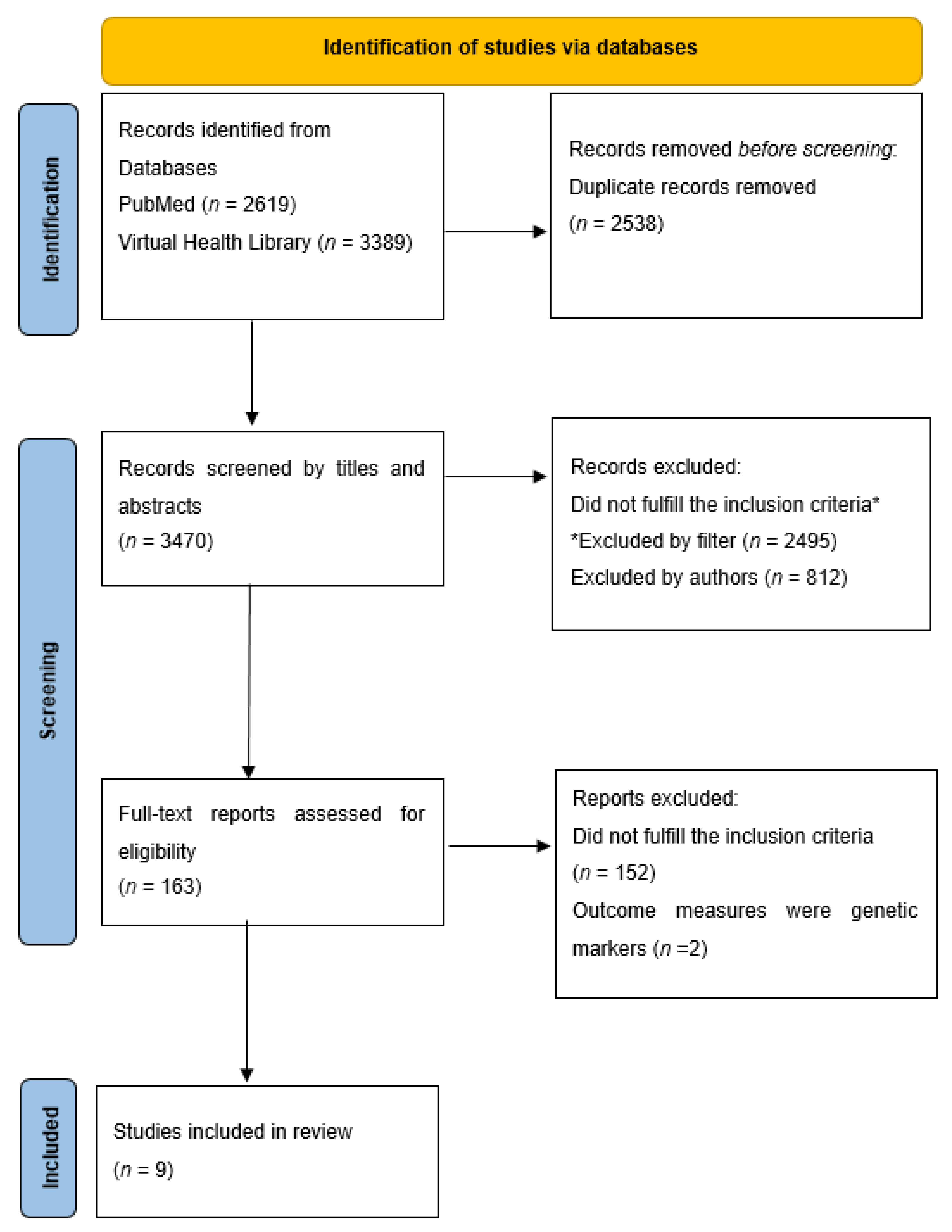
2. Materials and Methods
2.1. Study Design and Registration
2.2. Search Strategy
2.3. Eligibility Criteria
- Student Design—inclusion criteria were case-control studies, cross-sectional studies, or cohort studies.
- Population—subjects of the studies comprised adult (>18 years old) humans with RCD, including diagnosis of RC tendinopathy, shoulder impingement, subacromial bursitis, and RC tears. The diagnoses of RCD were confirmed by radiological or surgical examinations. Study populations involving other shoulder pathologies such as fractures, frozen shoulders, and shoulder joint dislocations were excluded. Subjects with other systemic conditions or comorbidities which could affect the outcomes were excluded. In addition, the studies were accompanied by suitable control groups without pathologies in the shoulders or without systemic conditions such as inflammatory joint disease and without immunological or neoplastic disorders. All animal studies were excluded.
- Outcomes—the studies needed to have outcome measures that could objectively quantify physiological biomarkers in blood samples of the subjects. Studies that lacked statistical analyses to compare their outcomes with were excluded.
2.4. Study Selection
2.5. Quality and Risk of Bias Assessment
2.6. Data Extraction
3. Results
3.1. Study Characteristics
3.2. Quality and Risk of Bias of Selected Articles
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
Appendix A.1. Search String on PubMed
Appendix A.2. Search String on Virtual Health Library
References
- Dang, A.; Davies, M. Rotator Cuff Disease: Treatment Options and Considerations. Sports Med. Arthrosc. 2018, 26, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Whittle, S.; Buchbinder, R. Rotator Cuff Disease. Ann. Intern. Med. 2015, 162, ITC1–ITC16. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, A.; Hurworth, M.; O’Sullivan, P.; Mitchell, T.; Smith, A. Rising trends in surgery for rotator cuff disease in Western Australia. ANZ J. Surg. 2016, 86, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Schairer, W.W.; Nwachukwu, B.U.; Fu, M.C.; Warren, R.F. Risk Factors for Short-term Complications After Rotator Cuff Repair in the United States. Arthrosc. J. Arthrosc. Relat. Surg. 2018, 34, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.J.F.; Franklin, S.L.; Carr, A.J. A systematic review of the histological and molecular changes in rotator cuff disease. Bone Jt. Res. 2013, 1, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Morita, W.; Dakin, S.G.; Snelling, S.J.B.; Carr, A.J. Cytokines in tendon disease. Bone Jt. Res. 2017, 6, 656–664. [Google Scholar] [CrossRef]
- Notarnicola, A.; Maccagnano, G.; Maresca, L.; Oliva, M.C.; Fari, G.; Papagni, G.; Pignatelli, G.; Covelli, I.; Gioia, G.; Bianchi, F.P.; et al. Is extracorporeal shockwave therapy effective even in the treatment of partial rotator cuff tear? J. Biol. Regul. Homeost. Agents 2020, 34, 709–714. [Google Scholar] [CrossRef]
- Burne, G.; Mansfield, M.; Gaida, J.E.; Lewis, J.S. Is there an association between metabolic syndrome and rotator cuff-related shoulder pain? A systematic review. BMJ Open Sport Exerc. Med. 2019, 5, e000544. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021); Cochrane. 2019. Available online: https://training.cochrane.org/handbook/current (accessed on 2 November 2021).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Longo, U.G.; Margiotti, K.; Petrillo, S.; Rizzello, G.; Fusilli, C.; Maffulli, N.; De Luca, A.; Denaro, V. Genetics of rotator cuff tears: No association of col5a1 gene in a case-control study. BMC Med. Genet. 2018, 19, 217. [Google Scholar] [CrossRef]
- Peach, C.A.; Zhang, Y.; Dunford, J.E.; Brown, M.A.; Carr, A.J. Cuff tear arthropathy: Evidence of functional variation in pyrophosphate metabolism genes. Clin. Orthop. Relat. Res. 2007, 462, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, Y.A.; Izaguirre, A.; Sierra, L.; Perez, F.; Cruz, F.; Villalobos, E.; Almazan, A.; Ibarra, C. Effect of Angiogenesis-Related Cytokines on Rotator Cuff Disease: The Search for Sensitive Biomarkers of Early Tendon Degeneration. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2011, 4, CMAMD.S7071. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, H.C.B.; Eliasson, P.; Aspenberg, P.; Adolfsson, L.E. Elevated plasma levels of TIMP-1 in patients with rotator cuff tear. Acta Orthop. 2012, 83, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The Levels of Evidence and Their Role in Evidence-Based Medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Lakemeier, S.; Reichelt, J.J.; Patzer, T.; Fuchs-Winkelmann, S.; Paletta, J.R.; Schofer, M.D. The association between retraction of the torn rotator cuff and increasing expression of hypoxia inducible factor 1α and vascular endothelial growth factor expression: An immunohistological study. BMC Musculoskelet. Disord. 2010, 11, 230. [Google Scholar] [CrossRef]
- Lakemeier, S.; Reichelt, J.J.; Timmesfeld, N.; Fuchs-Winkelmann, S.; Paletta, J.R.; Schofer, M.D. The relevance of long head biceps degeneration in the presence of rotator cuff tears. BMC Musculoskelet. Disord. 2010, 11, 191. [Google Scholar] [CrossRef][Green Version]
- Castagna, A.; Cesari, E.; Gigante, A.; Conti, M.; Garofalo, R. Metalloproteases and their inhibitors are altered in both torn and intact rotator cuff tendons. Musculoskelet. Surg. 2013, 97, 39–47. [Google Scholar] [CrossRef]
- Shindle, M.K.; Chen, C.C.T.; Robertson, C.; DiTullio, A.E.; Paulus, M.C.; Clinton, C.M.; Cordasco, F.A.; Rodeo, S.A.; Warren, R.F. Full-thickness supraspinatus tears are associated with more synovial inflammation and tissue degeneration than partial-thickness tears. J. Shoulder Elb. Surg. 2011, 20, 917–927. [Google Scholar] [CrossRef]
- Chaudhury, S.; Xia, Z.; Thakkar, D.; Hakimi, O.; Carr, A.J. Gene expression profiles of changes underlying different-sized human rotator cuff tendon tears. J. Shoulder Elb. Surg. 2016, 25, 1561–1570. [Google Scholar] [CrossRef]
- Jacob, J.; Eisemon, E.; Sheibani-Rad, S.; Patel, A.; Jacob, T.; Houeka, J. Matrix Metalloproteinase Levels as a Marker for Rotator Cuff Tears. Orthopedics 2012, 35, e474–e478. [Google Scholar] [CrossRef]
- Lehmann, L.; Schollmeyer, A.; Stoeve, J.; Scharf, H.-P. Biochemische Analyse der Synovialflüssigkeit bei Patienten mit und ohne Rotatorenmanschettendefekt. Z. Orthop. Unfall. 2009, 148, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Lo, I.K.Y.; Marchuk, L.L.; Hollinshead, R.; Hart, D.A.; Frank, C.B. Matrix Metalloproteinase and Tissue Inhibitor of Matrix Metalloproteinase mRNA Levels are Specifically Altered in Torn Rotator Cuff Tendons. Am. J. Sports Med. 2004, 32, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Blaine, T.A.; Cote, M.A.; Proto, A.; Mulcahey, M.; Lee, F.Y.; Bigliani, L.U. Interleukin-1β stimulates stromal-derived factor-1α expression in human subacromial bursa. J. Orthop. Res. 2011, 29, 1695–1699. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Hamada, K.; Yamakawa, H.; Nakamura, M.; Yamazaki, H.; Ueyama, Y.; Tamaoki, N.; Inoue, A.; Fukuda, H. Perforation of rotator cuff increases interleukin 1beta production in the synovium of glenohumeral joint in rotator cuff diseases. J. Rheumatol. 2000, 27, 2886–2892. [Google Scholar]
- Gotoh, M.; Hamada, K.; Yamakawa, H.; Yanagisawa, K.; Nakamura, M.; Yamazaki, H.; Inoue, A.; Fukuda, H. Interleukin-1-induced glenohumeral synovitis and shoulder pain in rotator cuff diseases. J. Orthop. Res. 2002, 20, 1365–1371. [Google Scholar] [CrossRef]
- Ko, J.; Wang, F.; Huang, H.; Wang, C.; Tseng, S.; Hsu, C. Increased IL-1β expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shoulder stiffness. J. Orthop. Res. 2008, 26, 1090–1097. [Google Scholar] [CrossRef]
- Joseph, M.; Maresh, C.M.; McCarthy, M.B.; Kraemer, W.J.; Ledgard, F.; Arciero, C.L.; Anderson, J.M.; Nindl, B.C.; Mazzocca, A.D. Histological and molecular analysis of the biceps tendon long head post-tenotomy. J. Orthop. Res. 2009, 27, 1379–1385. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Hamada, K.; Gotoh, M.; Tokunaga, T.; Oshika, Y.; Tomisawa, M.; Lee, Y.H.; Handa, A.; Kijima, H.; Yamazaki, H.; et al. Vascular endothelial growth factor (VEGF) expression in the subacromial bursa is increased in patients with impingement syndrome. J. Orthop. Res. 2001, 19, 448–455. [Google Scholar] [CrossRef]
- Millar, N.L.; Wei, A.Q.; Molloy, T.J.; Bonar, F.; Murrell, G.A.C. Cytokines and apoptosis in supraspinatus tendinopathy. J. Bone Jt. Surg. Br. 2009, 91-B, 417–424. [Google Scholar] [CrossRef]
- Millar, N.L.; Akbar, M.; Campbell, A.L.; Reilly, J.H.; Kerr, S.C.; McLean, M.; Frleta-Gilchrist, M.; Fazzi, U.G.; Leach, W.J.; Rooney, B.P.; et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep. 2016, 6, 27149. [Google Scholar] [CrossRef]
- Dakin, S.G.; Martinez, F.O.; Yapp, C.; Wells, G.; Oppermann, U.; Dean, B.J.F.; Smith, R.D.J.; Wheway, K.; Watkins, B.; Roche, L.; et al. Inflammation activation and resolution in human tendon disease. Sci. Transl. Med. 2015, 7, 311ra173. [Google Scholar] [CrossRef]
- Sakai, H.; Fujita, K.; Sakai, Y.; Mizuno, K. Immunolocalization of cytokines and growth factors in subacromial bursa of rotator cuff tear patients. Kobe J. Med. Sci. 2001, 47, 25–34. [Google Scholar] [PubMed]
- Wang, M.-X.; Wei, A.; Yuan, J.; Clippe, A.; Bernard, A.; Knoops, B.; Murrell, G.A.C. Antioxidant Enzyme Peroxiredoxin 5 Is Upregulated in Degenerative Human Tendon. Biochem. Biophys. Res. Commun. 2001, 284, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.T.; McDonnell, S.M.; Knowles, H.J.; Rees, J.L.; Carr, A.J.; Hulley, P.A. Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. J. Bone Jt. Surg. Br. 2010, 92-B, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.T.; McDonnell, S.M.; Rees, J.L.; Athanasou, N.A.; Carr, A.J. The morphological and immunocytochemical features of impingement syndrome and partial-thickness rotator-cuff tear in relation to outcome after subacromial decompression. J. Bone Jt. Surg. Br. 2009, 91-B, 119–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Millar, N.L.; Reilly, J.H.; Kerr, S.C.; Campbell, A.L.; Little, K.J.; Leach, W.J.; Rooney, B.P.; Murrell, G.A.C.; McInnes, I.B. Hypoxia: A critical regulator of early human tendinopathy. Ann. Rheum. Dis. 2012, 71, 302–310. [Google Scholar] [CrossRef]
- Lakemeier, S.; Braun, J.; Efe, T.; Foelsch, C.; Archontidou-Aprin, E.; Fuchs-Winkelmann, S.; Paletta, J.R.J.; Schofer, M.D. Expression of matrix metalloproteinases 1, 3, and 9 in differing extents of tendon retraction in the torn rotator cuff. Knee Surgery, Sport. Traumatol. Arthrosc. 2011, 19, 1760–1765. [Google Scholar] [CrossRef]
- Tillander, B.; Franzén, L.; Norlin, R. Fibronectin, MMP-1 and histologic changes in rotator cuff disease. J. Orthop. Res. 2002, 20, 1358–1364. [Google Scholar] [CrossRef]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef]
- Blaine, T.A.; Kim, Y.-S.; Voloshin, I.; Chen, D.; Murakami, K.; Chang, S.-S.; Winchester, R.; Lee, F.Y.; O’Keefe, R.J.; Bigliani, L.U. The molecular pathophysiology of subacromial bursitis in rotator cuff disease. J. Shoulder Elb. Surg. 2005, 14, S84–S89. [Google Scholar] [CrossRef]
- Lakemeier, S.; Schwuchow, S.A.; Peterlein, C.D.; Foelsch, C.; Fuchs-Winkelmann, S.; Archontidou-Aprin, E.; Paletta, J.R.; Schofer, M.D. Expression of matrix metalloproteinases 1, 3, and 9 in degenerated long head biceps tendon in the presence of rotator cuff tears: An immunohistological study. BMC Musculoskelet. Disord. 2010, 11, 271. [Google Scholar] [CrossRef]
- Osawa, T.; Shinozaki, T.; Takagishi, K. Multivariate analysis of biochemical markers in synovial fluid from the shoulder joint for diagnosis of rotator cuff tears. Rheumatol. Int. 2005, 25, 436–441. [Google Scholar] [CrossRef]
- Voloshin, I.; Gelinas, J.; Maloney, M.D.; O’Keefe, R.J.; Bigliani, L.U.; Blaine, T.A. Proinflammatory Cytokines and Metalloproteases Are Expressed in the Subacromial Bursa in Patients With Rotator Cuff Disease. Arthrosc. J. Arthrosc. Relat. Surg. 2005, 21, 1076.e1–1076.e9. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, S.; Carr, A.J. Lessons we can learn from gene expression patterns in rotator cuff tears and tendinopathies. J. Shoulder Elb. Surg. 2012, 21, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Mandl-Weber, S.; Cohen, C.D.; Haslinger, B.; Kretzler, M.; Sitter, T. Vascular endothelial growth factor production and regulation in human peritoneal mesothelial cells. Kidney Int. 2002, 61, 570–578. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rees, J.D.; Stride, M.; Scott, A. Tendons—Time to revisit inflammation. Br. J. Sports Med. 2014, 48, 1553–1557. [Google Scholar] [CrossRef]
- Tang, C.; Chen, Y.; Huang, J.; Zhao, K.; Chen, X.; Yin, Z.; Heng, B.C.; Chen, W.; Shen, W. The roles of inflammatory mediators and immunocytes in tendinopathy. J. Orthop. Transl. 2018, 14, 23–33. [Google Scholar] [CrossRef]
- Hoeben, A.; Landuyt, B.; Highley, M.S.; Wildiers, H.; Van Oosterom, A.T.; De Bruijn, E.A. Vascular Endothelial Growth Factor and Angiogenesis. Pharmacol. Rev. 2004, 56, 549–580. [Google Scholar] [CrossRef]
- Ucuzian, A.A.; Gassman, A.A.; East, A.T.; Greisler, H.P. Molecular Mediators of Angiogenesis. J. Burn Care Res. 2010, 31, 158–175. [Google Scholar] [CrossRef]
- Park, H.B.; Gwark, J.-Y.; Im, J.-H.; Jung, J.; Na, J.-B.; Yoon, C.H. Factors Associated with Atraumatic Posterosuperior Rotator Cuff Tears. J. Bone Jt. Surg. Am. 2018, 100, 1397–1405. [Google Scholar] [CrossRef]
- Hudek, R.; von Schacky, C.; Passow, A.; Abdelkawi, A.F.; Werner, B.; Gohlke, F. Degenerative rotator cuff tears are associated with a low Omega-3 Index. Prostaglandins Leukot. Essent. Fatty Acids 2019, 148, 35–40. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, G.-T.; Yoon, S.; Lee, H.I.; Ko, K.R.; Lee, S.-C.; Kim, D.K.; Shin, J.; Lee, S.-Y.; Lee, S. Low serum vitamin B12 levels are associated with degenerative rotator cuff tear. BMC Musculoskelet. Disord. 2021, 22, 364. [Google Scholar] [CrossRef]
- Papalia, R.; Del Buono, A.; Leonardi, F.; Osti, L.; Maffulli, N.; Denaro, V. Creatinine and nonprotein nitrogen plasma levels: Possible etiopathogenetic factors in rotator cuff tears. Phys. Sportsmed. 2011, 39, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Franceschi, F.; Ruzzini, L.; Spiezia, F.; Maffulli, N.; Denaro, V. Higher fasting plasma glucose levels within the normoglycaemic range and rotator cuff tears. Br. J. Sports Med. 2009, 43, 284–287. [Google Scholar] [CrossRef]
- Grusky, A.Z.; Song, A.; Kim, P.; Ayers, G.D.; Higgins, L.D.; Kuhn, J.E.; Baumgarten, K.M.; Matzkin, E.; Jain, N.B. Factors Associated With Symptomatic Rotator Cuff Tears. Am. J. Phys. Med. Rehabil. 2021, 100, 331–336. [Google Scholar] [CrossRef]
- Hedderson, W.C.; Borsa, P.A.; Fillingim, R.B.; Coombes, S.A.; Hass, C.J.; George, S.Z. Plasma Concentrations of Select Inflammatory Cytokines Predicts Pain Intensity 48 Hours Post-Shoulder Muscle Injury. Clin. J. Pain 2020, 36, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, I.; Lee, H.A.; Shin, S.-J. Factors Related to Symptomatic Failed Rotator Cuff Repair Leading to Revision Surgeries After Primary Arthroscopic Surgery. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.S.; Kim, H.-O.; Cheon, Y.-H.; Kim, M.; Kim, R.-B.; Park, K.-S.; Park, H.B.; Na, J.-B.; Moon, J.I.; Lee, S.-I. Metabolic and inflammatory links to rotator cuff tear in hand osteoarthritis: A cross sectional study. PLoS ONE 2020, 15, e0228779. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Mazzola, A.; Carotti, S.; Francesconi, M.; Catapano, S.; Magrì, F.; Perrone, G.; Morini, S.; De Salvatore, S.; Denaro, V. The role of estrogen and progesterone receptors in the rotator cuff disease: A retrospective cohort study. BMC Musculoskelet. Disord. 2021, 22, 891. [Google Scholar] [CrossRef]
- Hansen, M.; Kjaer, M. Sex Hormones and Tendon. In Metabolic Influences on Risk for Tendon Disorders. Advances in Experimental Medicine and Biology; Ackermann, P., Hart, D., Eds.; Springer: Cham, Switzerland, 2016; Volume 920, pp. 139–149. [Google Scholar] [CrossRef]
- Longo, U.G.; Candela, V.; Berton, A.; De Salvatore, S.; Fioravanti, S.; Giannone, L.; Marchetti, A.; De Marinis, M.G.; Denaro, V. Biosensors for Detection of Biochemical Markers Relevant to Osteoarthritis. Biosensors 2021, 11, 31. [Google Scholar] [CrossRef]
| Author | Study Design and Level of Evidence | Population and Diagnosis | Biomarkers | Results |
|---|---|---|---|---|
| Hallgren et al., 2012 | Case-Control Level III | Experimental group 17 patients with sonographically verified rotator cuff tears Mean age 61 (39–77) years Control group # 16 age and sex-matched individuals with no history of shoulder disease and sonographically intact rotator cuffs | Plasma samples MMP-1 MMP-2 MMP-3 MMP-7 MMP-9 TIMP-1 TIMP-2 TIMP-3 TIMP-4 | TIMP-1, median (range) Experimental group 86 (67–119) ng/mL Control group 78 (66–93) ng/mL Measured by Luminex, p = 0.04 Not significant when repeated using ELISA method, p = 0.2 Other measures not significant |
| Hudek et al., 2019 | Case-Control Level III | Experimental group 29 patients with non-traumatic complete rotator-cuff tears (22 males, 7 females; mean age: 53.9 ± 7.1 years) Diagnosis confirmed by MRI and surgery Control group 15 non-smoking subjects with healthy shoulders (10 males, 5 females; mean age 52.5 ± 6.5 years) | Plasma sample Fatty acids C16:1n-7 C20:2n-6 C24:0 C24:1n-9 C22:6n-3 Omega-3 Index | C16:1n-7 Exp: 0.59 ± 0.29 Control: 0.33 ± 0.12 * p < 0.01 C20:2n-6 Exp: 0.22 ± 0.04 Control: 0.19 ± 0.02 * p = 0.02 C24:0 Exp: 0.71 ± 0.24 Control: 0.52 ± 0.21 * p = 0.01 C24:1n-9 Exp: 0.78 ± 0.17 Control: 0.65 ± 0.20 * p = 0.04 C22:6n-3 Exp: 4.23 ± 1.11 Control: 5.10 ± 1.12 * p = 0.02 Omega-3 Index Exp: 5.01 ± 1.27 Control: 6.01 ± 1.39 * p = 0.03 |
| Park et al., 2018 | Cohort study Level III | Experiment group 199 patients with posterosuperior rotator cuff tears confirmed by MRI 143 patients were symptomatic 107 male (53.8%) Age 61.9 ± 7.6 Control group 435 individuals with intact RC 200 male (46.0%) Age 57.7 ± 8.8 | Serum lipid levels Total cholesterol LDL TG HDL Non-HDL | Experimental group Total cholesterol 200 ± 35 LDL 133 ± 33 TG 109 (83–60) HDL 54 (45–63) Non-HDL 145 ± 35 Control group Total cholesterol 198 ± 37 LDL 131 ± 34 TG 104 (78–150) HDL 57 (46–67) Non-HDL 140 ± 37 Unit: mg/dL HDL odds ratio = 0.99 (0.98–1.00), p = 0.035 Other measures not significant Dyslipidemia–Hypo-HDLemia Experimental group = 62 (31.2%) Control group = 75 (17.2%) Hypo-HDLemia odds ratio = 2.17 (1.47–3.21), * p < 0.001 |
| Longo et al., 2009 | Case-control Level III | Experimental group 97 patients with rotator cuff tears (36 men and 61 women; mean age: 62.9 years, range 37 to 82) Diagnosis was based on clinical and imaging grounds and surgery Control group 97 patients with no evidence of shoulder pathologies (36 men and 61 women; mean age: 61.6 years, range 36 to 80) | Venous fasting plasma glucose levels | Experimental group 99.17 ± 9.04 mg per decilitre 5.5 ± 0.5 millimoles per litre Control group 95.45 ± 9.87 mg per decilitre 5.3 ± 0.55 millimoles per litre * p < 0.01, both groups were within the normoglycemic range |
| Longo et al., 2010 | Case-control Level III | Experimental group 120 patients underwent arthroscopic repair of rotator cuff tears (45 men and 75 women; mean age: 64.86 years) Diagnosis was confirmed by MRI Control group 120 patients underwent knee arthroscopic meniscectomies with no evidence of shoulder pathologies (45 men and 75 women; mean age: 63.91 years) | Serum triglyceride and total cholesterol concentrations | Serum triglycerides Unit in mg per decilitre and millimoles per litre Experimental group Male 158.42 ± 122.29 1.81 ± 1.39 Female 131.81 ± 55.9 1.49 ± 0.63 Control group Male 139.87 ± 75.56 1.58 ± 0.85 Female 120.48 ± 53.75 1.36 ± 0.61 Serum cholesterol Unit in mg per decilitre and millimoles per litre Experimental group Male 212.76 ± 40.58 5.51 ± 1.05 Female 224.11 ± 44.42 5.80 ± 1.15 Control group Male 213.6 ± 36.45 5.53 ± 0.94 Female 217.3 ± 39.28 5.63 ± 1.02 not statistically significant differences either in triglyceride concentration (p = 0.6) or total cholesterol concentration (p = 0.1) |
| Longo et al., 2014 | Case-control Level III | Experimental group 82 patients underwent arthroscopic repair of RC tears (36 men and 46 women; mean age: 57.7 ± 10.2 years) Diagnosis confirmed by MRI Control group 82 patients underwent arthroscopic meniscectomies for meniscal tears with no history of RC symptoms (36 men and 46 women; mean age: 55.9 ± 9 years) | Serum fibrinogen concentration. | Experimental group 335.9 ± 171 mg/dL (range 70–512; median 328.5) Control group 329.6 ± 205 mg/dL (range 72–607; median 322.5) p = 0.05 |
| Papalia et al., 2011 | Case-control Level III | Experimental group 200 patients who underwent arthroscopic repair of rotator cuff tears (93 men, 107 women; age 56.8 ±11.7 years) Diagnosis was confirmed by preoperative imaging and arthroscopy Control group 200 patients who underwent knee arthroscopies for management of meniscal tears, with or without cartilage damage (93 men and 107 women, age of 53.9 ± 12.6 years) | Plasma Nonprotein nitrogen (NPN) and creatinine levels | NPN Experimental group 37.2 ± 8.9 mg/dL 2.06 ± 0.49 mmol/L Control group 35.9 ± 10.2 mg/dL 1.98 ± 0.56 mmol/L * p = 0.035 Creatinine Experimental group 0.8 ± 0.19 mg/dL 0.04 ± 0.01 mmol/L Control group 0.82 ± 0.18 mg/dL 0.045 ± 0.01 mmol/L p = 0.66 |
| Savitskaya et al., 2011 | Case-control Level III | Experimental group 200 patients with significant inflammation, tendon degeneration, and partial or full-thickness rotator cuff tears (112 males, 88 females, age 40.3 ± 10.9) Diagnosis was confirmed by MRI Control group 200 age and sex matched healthy individuals with no medical history of rotator cuff disease (107 males, 93 females, age 43.3 ± 11.5) | Serum samples IL-1β IL-8 IL-10 VEGF ANG | Experimental group IL-1 16.17 ± 6.71–43.71 ± 8.91 IL-8 15.31 ± 0.85–27.81 ± 1.11 IL-10 3.11 ± 1.91–7.64 ± 1.11 VGEF 402.11 ± 88.11–621.24 ± 301.11 ANG 166.45 ± 44.90–89.39–40.19 Control group IL-1 3.33 ± 0.69 IL-8 9.11 ± 0.98 IL-10 9.53 ± 1.21 VEGF 339.67 ± 74.65 ANG 239.51 ± 58.4 Unit in pg/mL * IL-1β, IL-8, and VEGF levels were significantly higher in RCD patients than in controls. Serum ANG and IL-10 levels were significantly lower in RCD patients than in controls Exact p values not provided * Overexpression of VEGF correlated with advanced disease (r = 0.75; p < 0.01), average microvascular density (r = 0.68, p < 0.01), and visual analog score (r = 0.75, p < 0.01) in patients with RCD. |
| Kim et al., 2021 | Case-control Level III | Experimental group 40 patients with degenerative RC tears Diagnosis was confirmed by MRI (23 males, 17 females, age 61.0 ± 5.3) Control group (n = 47) 40 patients with minor non-shoulder trauma but no RC tears or associated symptoms or clinical signs | Serum sample Glucose Magnesium Calcium Phosphorus Zinc Homocysteine Vitamin D Vitamin B12 Folate | Vitamin B12 Experimental group 528.4 ± 145.7 pg/mL Control group 627.1 ± 183.0 pg/mL * p = 0.007 Vitamin D Experimental group 15.7 ± 7.2 ng/mL Control group 21.6 ± 10.0 ng/mL * p = 0.002 Vit D odds ratio (OR) for degenerative RC tear = 0.89; 95% CI = 0.82–0.96; * p = 0.006 Phosphorus Experimental group 3.2 ± 0.6 mg/dL Control group 3.6 ± 0.7 mg/dL * p = 0.008 other parameters showed no significant relationships |
| Hallgren et al., 2012 | Hudek et al., 2019 | Park et al., 2018 | Longo et al., 2009 | Longo et al., 2010 | Longo et al., 2014 | Papalia et al., 2011 | Savitskaya et al., 2011 | Kim et al., 2021 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 2. Was the study population clearly specified and defined? | Y | Y | N | Y | N | N | Y | N | Y |
| 3. Was the participation rate of eligible persons at least 50%? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Y | Y | Y | Y | Y | Y | Y | N | Y |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | N | Y | N | N | N | N | Y | N | Y |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | N | N | N | N | N | N | N | N | N |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | N | N | N | N | N | N | N | N | N |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 10. Was the exposure(s) assessed more than once over time? | N | N | N | N | N | N | N | N | N |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 12. Were the outcome assessors blinded to the exposure status of participants? | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 13. Was loss to follow-up after baseline 20% or less? | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | N | N | N | Y | N | N | Y | N | Y |
| Number of “yes” | 7 | 8 | 6 | 8 | 6 | 6 | 9 | 5 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo, C.N.; Leung, B.P.L.; Ngai, S.P.C. The Usefulness of Serological Inflammatory Markers in Patients with Rotator Cuff Disease—A Systematic Review. Medicina 2022, 58, 301. https://doi.org/10.3390/medicina58020301
Lo CN, Leung BPL, Ngai SPC. The Usefulness of Serological Inflammatory Markers in Patients with Rotator Cuff Disease—A Systematic Review. Medicina. 2022; 58(2):301. https://doi.org/10.3390/medicina58020301
Chicago/Turabian StyleLo, Chi Ngai, Bernard Pui Lam Leung, and Shirley Pui Ching Ngai. 2022. "The Usefulness of Serological Inflammatory Markers in Patients with Rotator Cuff Disease—A Systematic Review" Medicina 58, no. 2: 301. https://doi.org/10.3390/medicina58020301
APA StyleLo, C. N., Leung, B. P. L., & Ngai, S. P. C. (2022). The Usefulness of Serological Inflammatory Markers in Patients with Rotator Cuff Disease—A Systematic Review. Medicina, 58(2), 301. https://doi.org/10.3390/medicina58020301






