Status of Glucocorticoid-Induced Osteoporosis Preventive Care in Korea: A Retrospective Cohort Study on the Korean National Health Insurance Service Database
Abstract
:1. Introduction
2. Materials and Methods
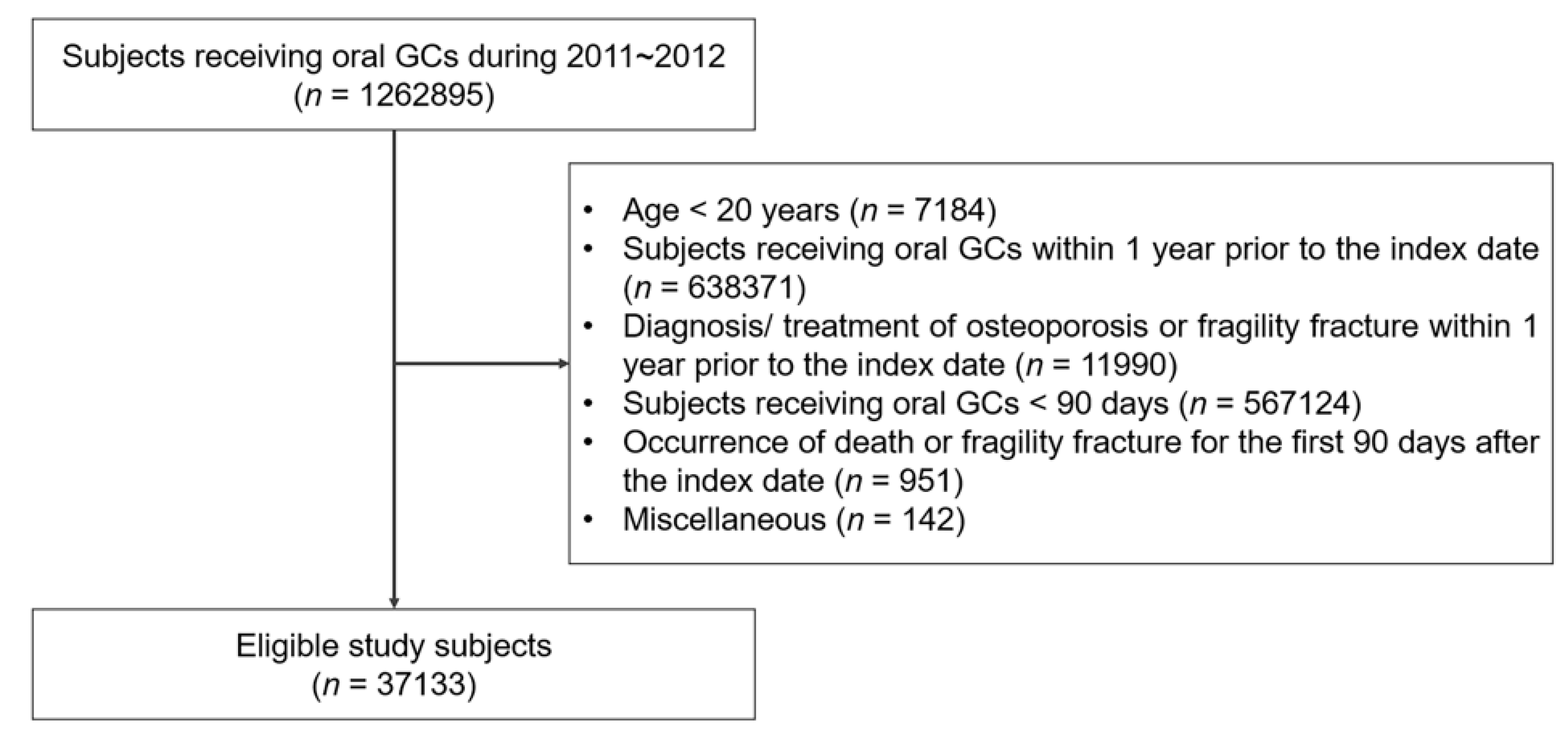
2.1. Study Design and Subjects
2.2. Outcomes
2.3. Covariates
2.4. Statistical Analyses
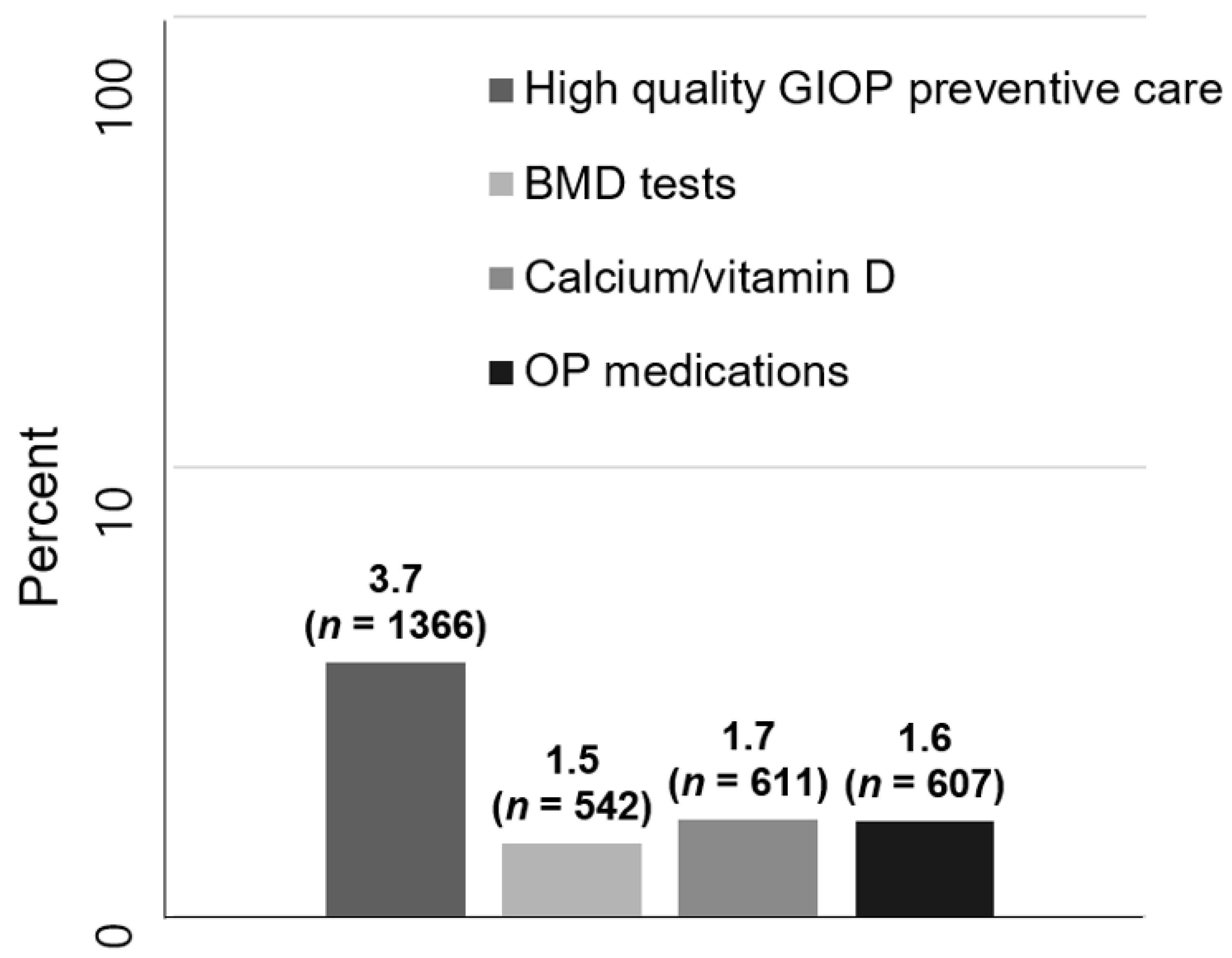
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobza, A.O.; Herman, D.; Papaioannou, A.; Lau, A.N.; Adachi, J.D. Understanding and Managing Corticosteroid-Induced Osteoporosis. Open Access Rheumatol. 2021, 13, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S.; Scaturro, D.; Santoro, M.; Di Gaetano, G.; Vitagliani, F.; Falco, V.; Siragusa, S.; Gonnelli, S.; Mauro, G.L. Bone damage after chemotherapy for lymphoma: A real-world experience. BMC Musculoskelet. Disord. 2021, 22, 1024. [Google Scholar] [CrossRef] [PubMed]
- Albaum, J.M.; Levesque, L.E.; Gershon, A.S.; Liu, G.; Cadarette, S.M. Glucocorticoid-induced osteoporosis management among seniors, by year, sex, and indication, 1996–2012. Osteoporos. Int. 2015, 26, 2845–2852. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.-H. Korean Guideline of Glucocorticoid-induced Osteoporosis; Time to Prevent Fracture! J. Rheum. Dis. 2019, 26, 87–89. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Gong, H.S.; Kim, K.M.; Kim, D.; Kim, H.; Jeon, C.H.; Ju, J.H.; Lee, S.-S.; Park, D.A.; Sung, Y.-K.; et al. Korean Guideline for the Prevention and Treatment of Glucocorticoid-induced Osteoporosis. J. Rheum. Dis. 2018, 25, 263–295. [Google Scholar] [CrossRef] [Green Version]
- Buckley, L.; Guyatt, G.; Fink, H.A.; Cannon, M.; Grossman, J.; Hansen, K.E.; Humphrey, M.B.; Lane, N.E.; Magrey, M.; Miller, M.; et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Care Res. 2017, 69, 1095–1110. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nawata, H.; Soen, S.; Fujiwara, S.; Nakayama, H.; Tanaka, I.; Ozono, K.; Sagawa, A.; Takayanagi, R.; Tanaka, H.; et al. Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research: 2014 update. J. Bone Miner. Metab. 2014, 32, 337–350. [Google Scholar] [CrossRef]
- Briot, K.; Cortet, B.; Roux, C.; Fardet, L.; Abitbol, V.; Bacchetta, J.; Buchon, D.; Debiais, F.; Guggenbuhl, P.; Laroche, M.; et al. 2014 update of recommendations on the prevention and treatment of glucocorticoid-induced osteoporosis. Jt. Bone Spine 2014, 81, 493–501. [Google Scholar] [CrossRef]
- Lekamwasam, S.; Adachi, J.D.; Agnusdei, D.; Bilezikian, J.; Boonen, S.; Borgstrom, F.; Cooper, C.; Diez Perez, A.; Eastell, R.; Hofbauer, L.C.; et al. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos. Int. 2012, 23, 2257–2276. [Google Scholar] [CrossRef]
- Aagaard, E.M.; Lin, P.; Modin, G.W.; Lane, N.E. Prevention of glucocorticoid-induced osteoporosis: Provider practice at an urban county hospital. Am. J. Med. 1999, 107, 456–460. [Google Scholar] [CrossRef]
- Ettinger, B.; Chidambaran, P.; Pressman, A. Prevalence and determinants of osteoporosis drug prescription among patients with high exposure to glucocorticoid drugs. Am. J. Manag. Care 2001, 7, 597–605. [Google Scholar] [PubMed]
- Feldstein, A.C.; Elmer, P.J.; Nichols, G.A.; Herson, M. Practice patterns in patients at risk for glucocorticoid-induced osteoporosis. Osteoporos. Int. 2005, 16, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Westfall, A.O.; Allison, J.J.; Becker, A.; Casebeer, L.; Freeman, A.; Spettell, C.M.; Weissman, N.W.; Wilke, S.; Saag, K.G. Longitudinal patterns in the prevention of osteoporosis in glucocorticoid-treated patients. Arthritis Care Res. 2005, 52, 2485–2494. [Google Scholar] [CrossRef] [PubMed]
- Cruse, L.M.; Valeriano, J.; Vasey, F.B.; Carter, J.D. Prevalence of evaluation and treatment of glucocorticoid-induced osteoporosis in men. J. Clin. Rheumatol. 2006, 12, 221–225. [Google Scholar] [CrossRef]
- Che, M.; Ettinger, B.; Nguyen, M.T.; Pressman, A.R.; Johnston, J. High-dose corticosteroid exposure and osteoporosis intervention in adults. Ann. Allergy Asthma Immunol. 2006, 97, 497–501. [Google Scholar] [CrossRef]
- Saag, K.G.; Gehlbach, S.H.; Curtis, J.R.; Youket, T.E.; Worley, K.; Lange, J.L. Trends in prevention of glucocorticoid-induced osteoporosis. J. Rheumatol. 2006, 33, 1651–1657. [Google Scholar]
- Majumdar, S.R.; Lix, L.M.; Yogendran, M.; Morin, S.N.; Metge, C.J.; Leslie, W.D. Population-based trends in osteoporosis management after new initiations of long-term systemic glucocorticoids (1998–2008). J. Clin. Endocrinol. Metab. 2012, 97, 1236–1242. [Google Scholar] [CrossRef] [Green Version]
- Trijau, S.; de Lamotte, G.; Pradel, V.; Natali, F.; Allaria-Lapierre, V.; Coudert, H.; Pham, T.; Sciortino, V.; Lafforgue, P. Osteoporosis prevention among chronic glucocorticoid users: Results from a public health insurance database. RMD Open 2016, 2, e000249. [Google Scholar] [CrossRef] [Green Version]
- Soen, S.; Kaku, M.; Okubo, N.; Touzeni, S.; Saito, K.; Kobayashi, M. Epidemiology of glucocorticoid-induced osteoporosis and management of associated fracture risk in Japan. J. Bone Miner. Metab. 2021, 39, 1019–1030. [Google Scholar] [CrossRef]
- Lee, Y.K.; Jang, S.; Jang, S.; Lee, H.J.; Park, C.; Ha, Y.C.; Kim, D.Y. Mortality after vertebral fracture in Korea: Analysis of the National Claim Registry. Osteoporos. Int. 2012, 23, 1859–1865. [Google Scholar] [CrossRef]
- Kim, D.; Cho, S.K.; Park, B.; Jang, E.J.; Bae, S.C.; Sung, Y.K. Glucocorticoids Are Associated with an Increased Risk for Vertebral Fracture in Patients with Rheumatoid Arthritis. J. Rheumatol. 2018, 45, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.K.; Park, C.; Jang, S.; Jang, S.; Lee, Y.K.; Ha, Y.C. Incidence and mortality following hip fracture in Korea. J. Korean Med. Sci. 2011, 26, 1087–1092. [Google Scholar] [CrossRef] [Green Version]
- Kwon, G.D.; Jang, S.; Lee, A.; Park, C.M.; Lee, Y.K.; Kim, T.Y.; Kim, H.Y.; Park, E.J.; Ha, Y.C. Incidence and Mortality after Distal Radius Fractures in Adults Aged 50 Years and Older in Korea. J. Korean Med. Sci. 2016, 31, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.R.; Lix, L.M.; Morin, S.N.; Yogendran, M.; Metge, C.J.; Leslie, W.D. The disconnect between better quality of glucocorticoid-induced osteoporosis preventive care and better outcomes: A population-based cohort study. J. Rheumatol. 2013, 40, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.; Nanes, M. Advances in treatment of glucocorticoid-induced osteoporosis. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 411–417. [Google Scholar] [CrossRef]
| New GC Users (n = 37,133) | |
|---|---|
| Age | |
| <40 years, n (%) | 10,307 (27.8) |
| 40~49 years, n (%) | 7818 (21.1) |
| 50~59 years, n (%) | 8798 (23.7) |
| 60~69 years, n (%) | 5839 (15.7) |
| ≥70 years, n (%) | 4371 (11.8) |
| Age, years, mean ± SD | 49.8 ± 15.3 |
| Female, n (%) | 18,476 (49.8) |
| Residence | |
| Urban, n (%) | 33,899 (91.3) |
| Rural, n (%) | 3234 (8.7) |
| Institution | |
| Tertiary/general hospital, n (%) | 5861 (15.8) |
| Primary care clinic/nursing hospital, n (%) | 31,272 (84.2) |
| Initial GC prescriber specialty | |
| Rheumatologist, n (%) | 415 (1.1) |
| Non-rheumatologist internist, n (%) | 11,608 (31.3) |
| Non-internist, n (%) | 25,110 (67.6) |
| GC-requiring conditions | |
| Systemic autoimmune diseases, n (%) | 1274 (3.4) |
| Chronic pulmonary diseases, n (%) | 3171 (8.5) |
| Others, n (%) | 32,688 (88.1) |
| Cumulative GC dose *, mg, mean ± SD | 203.8 ± 151.4 |
| Comorbidities | |
| Hyperparathyroidism, n (%) | 8 (0) |
| Hyperthyroidism, n (%) | 579 (1.6) |
| Hypothyroidism, n (%) | 929 (2.5) |
| Malignancy, n (%) | 1127 (3) |
| No High-Quality GIOP Preventive Care (n = 35,767) | High-Quality GIOP Preventive Care (n = 1366) | p | |
|---|---|---|---|
| Age | |||
| <40 years, n (%) | 10,187 (28.5) | 120 (8.8) | <0.001 |
| 40~49 years, n (%) | 7588 (21.2) | 230 (16.8) | |
| 50~59 years, n (%) | 8415 (23.5) | 383 (28) | |
| 60~69 years, n (%) | 5545 (15.5) | 294 (21.5) | |
| ≥70 years, n (%) | 4032 (11.3) | 339 (24.8) | |
| Age, years, mean ± SD | 49.5 ± 15.3 | 58.4 ± 13.8 | <0.001 |
| Female, n (%) | 17,443 (48.8) | 1033 (75.6) | <0.001 |
| Residence | |||
| Urban, n (%) | 32,696 (91.4) | 1203 (88.1) | <0.001 |
| Rural, n (%) | 3071 (8.6) | 163 (11.9) | |
| Institution | |||
| Tertiary/general hospital, n (%) | 5513 (15.4) | 348 (25.5) | <0.001 |
| Primary care clinic/nursing hospital, n (%) | 30,254 (84.6) | 1018 (74.5) | |
| Initial GC prescriber specialty | |||
| Rheumatologist, n (%) | 374 (1) | 41 (3) | <0.001 |
| Non-rheumatologist internist, n (%) | 11,145 (31.2) | 463 (33.9) | |
| Non-internist, n (%) | 24,248 (67.8) | 862 (63.1) | |
| GC requiring conditions | |||
| Systemic autoimmune diseases, n (%) | 1124 (3.1) | 150 (11) | <0.001 |
| Chronic pulmonary diseases, n (%) | 3030 (8.5) | 141 (10.3) | |
| Others, n (%) | 31,613 (88.4) | 1075 (78.7) | |
| Cumulative GC dose *, mg, mean ± SD | 204.4 ± 150 | 190.1 ± 182.9 | 0.005 |
| Comorbidities | |||
| Hyperparathyroidism, n (%) | 8 (0) | 0 (0) | 0.58 |
| Hyperthyroidism, n (%) | 538 (1.5) | 41 (3) | <0.001 |
| Hypothyroidism, n (%) | 874 (2.4) | 55 (4) | <0.001 |
| Malignancy, n (%) | 1046 (2.9) | 81 (5.9) | <0.001 |
| Crude OR (95% CI) | p | Adjusted OR (95% CI) | p | |
|---|---|---|---|---|
| Age | ||||
| <40 years | 1 (ref.) | 1 (ref.) | ||
| 40~49 years | 2.57 (2.06–3.22) | <0.001 | 2.53 (2.02–3.17) | <0.001 |
| 50~59 years | 3.86 (3.14–4.75) | <0.001 | 3.99 (3.23–4.92) | <0.001 |
| 60~69 years | 4.5 (3.63–5.58) | <0.001 | 5.17 (4.16–6.43) | <0.001 |
| ≥70 years | 7.14 (5.78–8.82) | <0.001 | 8.07 (6.5–10.03) | <0.001 |
| Male | 0.31 (0.27–0.35) | <0.001 | 0.26 (0.23–0.3) | <0.001 |
| Residence | ||||
| Urban | 1 (ref.) | 1 (ref.) | ||
| Rural | 1.44 (1.22–1.71) | <0.001 | 1.19 (1–1.42) | 0.046 |
| Institution | ||||
| Tertiary/general hospital | 1 (ref.) | 1 (ref.) | ||
| Primary care clinic/nursing hospital | 0.53 (0.23–0.45) | <0.001 | 0.66 (0.57–0.75) | <0.001 |
| Initial GC prescriber specialty | ||||
| Rheumatologist | 1 (ref.) | 1 (ref.) | ||
| Non-rheumatologist internist | 0.38 (0.27–0.53) | <0.001 | 1.07 (0.73–1.59) | 0.722 |
| Non-internist | 0.32 (0.23–0.45) | <0.001 | 1.04 (0.7–1.53) | 0.854 |
| GC-requiring conditions | ||||
| Others | 1 (ref.) | 1 (ref.) | ||
| Systemic autoimmune diseases | 3.93 (3.28–4.7) | <0.001 | 3.08 (2.49–3.8) | <0.001 |
| Chronic pulmonary diseases | 1.37 (1.14–1.64) | 0.001 | 1.04 (0.86–1.26) | 0.708 |
| Cumulative GC dose *, g | 0.99 (0.99–1) | 0.001 | 1.25 (0.91–1.71) | 0.075 |
| Comorbidities | ||||
| Hyperparathyroidism | N/A | 0.957 | - | - |
| Hyperthyroidism | 2.03 (1.47–2.8) | <0.001 | 1.58 (1.13–2.21) | 0.007 |
| Hypothyroidism | 1.68 (1.27–2.22) | <0.001 | 1.06 (0.8–1.42) | 0.674 |
| Malignancy | 2.09 (1.66–2.64) | <0.001 | 1.59 (1.24–2.03) | <0.001 |
| Country | Definition of Long-Term GC Use | BMD Test | Calcium/Vitamin D | Osteoporosis Medications | Associated Factor for GIOP Prevention | |
|---|---|---|---|---|---|---|
| Albaum et al. [3] (n = 168,074) | Canada | Greater than or equal to two oral GC prescriptions dispensed and ≥450mg prednisolone equivalent over 6 months period | 7% for men, 13% for women | 12% for men, 30% for women | ||
| Aagaard et al. [10] (n = 215) | USA | Prednisone (or its equivalent) at a daily dose of at least 5 mg for at least 1 month | Calcium 42%, vitamin D 37% | 4% | Female ↑, aging ↑, rheumatologist ↑, more comorbid illness ↑, multiple medications ↑ | |
| Ettinger et al. [11] (n = 8807) | USA | Prescriptions for ≥2 g of prednisone (or equivalent) during any 12-month period | 8.8% | Female ↑, aging ↑, higher GC exposure ↑, rheumatologist ↑, previous osteoporotic fracture ↑ | ||
| Feldstein et al. [12] (n = 3031) | USA | Equivalent of >5 mg of prednisone per day for at least 90 days | 9.8% | 38% | ||
| Curtis et al. [13] (n = 6281) | USA | Outpatient oral GC treatment on at least 60 days | 33% | 69% | 37% | Female ↑, rheumatologist ↑, gastroenterologist ↓ |
| Cruse et al. [14] (n = 370) | USA | Long-term oral prednisone use | 44% | Calcium 51%, vitamin D 44% | 24% | |
| Saag et al. [16] (n = 3125) | USA | ≥7.5 mg/day of prednisone equivalent for >6 months | 10~19% | 50% | Female ↑, aging ↑, rheumatologist ↑ | |
| Majumdar et al. [17] (n = 17,736) | Canada | ≥ 90 days of GC use | 6% | 22% | Female ↑, aging ↑, rheumatologist ↑ | |
| Trijau et al. [18] (n = 32,812) | France | ≥7.5 mg of prednisone equivalent per day during at least 90 days | 8% | 18% | 12% | Female ↑, aging ↑, rheumatologist ↑, gastroenterologist ↑, internist ↑, higher mean GCc dose ↑, RA ↑, autoimmune disease ↑, IBD ↑ |
| Soen et al. [19] (n = 25,569) | Japan | GIOP risk score ≥ 3 | 51.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.-W.; Kim, A.-R.; Kim, M.-A.; Kim, H.-S.; Lee, S.-G. Status of Glucocorticoid-Induced Osteoporosis Preventive Care in Korea: A Retrospective Cohort Study on the Korean National Health Insurance Service Database. Medicina 2022, 58, 324. https://doi.org/10.3390/medicina58020324
Song B-W, Kim A-R, Kim M-A, Kim H-S, Lee S-G. Status of Glucocorticoid-Induced Osteoporosis Preventive Care in Korea: A Retrospective Cohort Study on the Korean National Health Insurance Service Database. Medicina. 2022; 58(2):324. https://doi.org/10.3390/medicina58020324
Chicago/Turabian StyleSong, Byung-Wook, A-Ran Kim, Min-A Kim, Ho-Seob Kim, and Seung-Geun Lee. 2022. "Status of Glucocorticoid-Induced Osteoporosis Preventive Care in Korea: A Retrospective Cohort Study on the Korean National Health Insurance Service Database" Medicina 58, no. 2: 324. https://doi.org/10.3390/medicina58020324
APA StyleSong, B.-W., Kim, A.-R., Kim, M.-A., Kim, H.-S., & Lee, S.-G. (2022). Status of Glucocorticoid-Induced Osteoporosis Preventive Care in Korea: A Retrospective Cohort Study on the Korean National Health Insurance Service Database. Medicina, 58(2), 324. https://doi.org/10.3390/medicina58020324






